Abstract
Even now, only a portion of leukodystrophy patients are correctly diagnosed, though various causative genes have been identified. In the present report, we describe a case of adult-onset leukodystrophy in a woman with ovarian failure. By whole-exome sequencing, a compound heterozygous mutation consisting of NM_020745.3 (AARS2_v001):c.1145C>A and NM_020745.3 (AARS2_v001):c.2255+1G>A was identified. Neither of the mutations has been previously reported, and this is the first report of alanyl-transfer RNA synthetase 2 mutation in Asia. We anticipate that further studies of the molecular basis of leukodystrophy will provide insight into its pathogenesis and hopefully lead to sophisticated diagnostic and treatment strategies.
Similar content being viewed by others
Introduction
Leukodystrophy is a syndrome with progressive white matter degeneration. Its pathogenic background and clinical presentation are variable. Genetic rather than environmental factors are associated with its variablity.1 More than a hundred subtypes of leukodystrophy have been reported, and many causative genes have been identified.2 However, the heterogeneity and complexity of leukodystrophy make a definitive diagnosis difficult. Indeed, only half of leukodystrophy patients receive a specific diagnosis.3
Here, we report a Japanese woman presenting with adult-onset leukodystrophy and ovarian failure in her thirties. By whole-exome sequencing (WES), we detected novel compound heterozygous mutations in alanyl-transfer RNA (tRNA) synthetase 2 (AARS2) (OMIM *612035).
Case report
A 31-year-old Japanese woman first visited our hospital in 2010. She was born healthy with a normal delivery to non-consanguineous parents. She developed normally and graduated from college. She worked until she was 30 years old, when she suddenly quit her job and had reduced interactions with her surroundings. She developed cognitive decline and began abnormal behaviors (for example, buying the same thing on consecutive days). She had difficulty using her left limbs, and she tended to lean to the left while sitting. She became totally incontinent. No similar symptoms occurred in her parents or her older sister.
A general examination at age 33 revealed secondary amenorrhea, although she had normal menstrual cycles until then with normal secondary sexual characteristics. The unelevated gonadotropin level indicated the impairment of reactive gonadotropin release by hypothalamus and pituitary, suggesting the secondary ovarian failure. She also had generalized atopic dermatitis.
Neurological examination revealed significant decline in frontal lobe function. The patient had a Mini-Mental State Examination score of 19/30 and a Frontal Assessment Battery score of 4/18. Left hand ideomotor apraxia, hyperreflexia, and rigidity of neck and limbs were observed more on the left side. At the most recent examination at age 38, the patient was aphasic and almost bedridden.
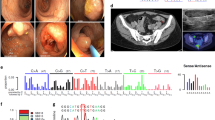
Brain magnetic resonance imaging (MRI) showed diffuse cerebral white matter abnormalities without gadolinium enhancement. Patchy, diffusion-restricted areas were present in the abnormal white matter. Thinning of corpus callosum was also observed (Figure 1). Cardiac ultrasound and electrocardiography showed no abnormalities.
Brain magnetic resonance imaging (MRI) of the patient at age 33. The fluid-attenuated inversion recovery (FLAIR) images show periventricular white matter abnormalities predominant at the parietal and frontal area, which revealed low signal lesions in the T1-weighted image (arrows in a, b and c). The diffusion-weighted image shows patchy areas of restricted diffusion in the abnormal white matter, which are confirmed by low signal of the corresponding areas on the apparent diffusion coefficient map (arrows in d, e and f). The sagittal FLAIR image shows thinned corpus callosum, especially in the splenium (arrow in g) and affected white matter structures in a tract-like manner (arrow in h). The MR angiography showed no stenosis of the major vessels (i).
Laboratory workups including plasma/cerebrospinal fluid lactate, serum arylsulfatase A, galactocerebrosidase, very long chain fatty acids and cholestanol were within normal ranges. Gene analyses of colony stimulating factor 1 receptor and eukaryotic translation initiation factor 2B were normal. We performed WES on genomic DNA.
Genetic analysis
Genetic analysis by WES was approved by the Tokyo Women’s Medical University Ethical Committee, and written informed consent was obtained from the patient’s parents. DNA samples from the patient and her parents were analyzed by WES, as previously described.4 Detailed methods of WES and prioritization of variants are described in the Supplementary Information.
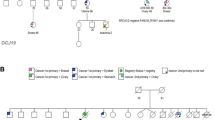
We focused on de novo and recessive mutations. We found novel compound heterozygous mutations in AARS2, NM_020745.3 (AARS2_v001):c.1145C>A and NM_020745.3 (AARS2_v001):c.2255+1G>A. These were the missense mutation (p.T382K) and the splice-site mutation, respectively. These two mutations were not registered in the dbSNP137, NHLBI-ESP 6500, HGVD or our in-house 575 Japanese control exome databases. Sanger sequencing confirmed that the mutations were transmitted maternally and paternally, respectively (Figure 2), and the in silico analysis for the pathogenicities is described in the Supplementary Information.
Next, we performed messenger RNA (mRNA) analysis around the segments harboring each mutation (Figure 3a). We could not detect any aberrantly-spliced mRNA caused by the splice-site mutation (Figure 3b). Direct sequence analysis of cDNA revealed that the patient was heterozygous at the locus for c.1145, and the sequence around c.2255 was normal without any aberrant insertion or truncation (Figure 3c).
Messenger RNA (mRNA) analysis. (a) Genomic structure of AARS2 was illustrated. Arrowheads indicate primer pairs. Triplets under bases indicate reading frames, and assuming that the mutation c.2255+1G>A causes splicing error, a premature stop codon will appear just afterwards (blue). (b) Image of PCR products of complementary DNA (cDNA) including each mutation (PCR1 and PCR2). (c) Direct sequence analysis of patient’s cDNA.
Discussion
The causative gene in a Japanese leukodystrophy patient with ovarian failure was identified as a compound heterozygous AARS2 mutation.
We initially considered the patient’s diagnosis as hereditary diffuse leukoencephalopathy with axonal spheroids, which is most prevalent in women in their 30 s and 40 s, and frequently includes corpus callosum thinning.5, 6, 7 We also suspected vanishing white matter disease, which includes ovarian failure as one of its features.8, 9, 10, 11 However, no mutation in causative genes for these diseases were identified.
Therefore, we performed a comprehensive genetic workup, and by use of WES, compound heterozygous mutations, c.1145C>A (p.T382K) and c.2255+1G>A, were detected. These mutations were inherited as an autosomal recessive trait. Both mutations were novel and predicted as pathogenic in silico. In the analysis of the patient’s mRNA, we could not detect any misspliced mature mRNA caused by the splice-site mutation, c.2255+1G>A. However, it is predicted that the mutation introduced a premature stop codon when the splicing at the exon16-intron16 boundary failed (Figure 3a). Then this transcript would be a target for the nonsense-mediated mRNA decay and should be degraded.
AARS2 encodes mitochondrial alanyl-tRNA synthetase, an enzyme that charges a specific tRNA with its cognate amino acid, alanine. AARS2 mutation was recently shown to cause mitochondrial dysfunction and reported in six patients with adult-onset leukodystrophy.12 In that report, two specific clinical phenotypes were described: characteristic MRI features and ovarian failure. Common MRI features are striking white matter tract involvement and presence of spots of restricted diffusion in the cerebral white matter. Corpus callosum thinning and the strip of signal abnormality in the splenium were also seen. Ovarian failure was present in all of the previously reported five female cases; one case had secondary amenorrhea like the present patient and four cases had primary amenorrhea.
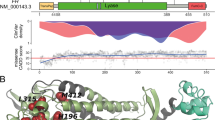
AARS2 mutations have another subtype, severe infantile cardiomyopathy with early fatal outcome.13, 14, 15 In contrast to this infantile subtype, the later-onset subtype, which includes the present case, has no signs of cardiomyopathy. Therefore, the question arises why AARS2 mutations cause two very different subtypes with dissimilar tissue involvement. In 2015, Euro et al.16 gave the elegant explanation for the phenomenon. AARS2 is unique among the human mitochondrial aminoacyl-tRNA synthetases because it contains an editing domain for deacylating mischarged tRNAs in addition to the aminoacylation domain. All patients with infantile-onset cardiomyopathy had the c.1774C>T (p.R592W) mutation located in the editing domain in at least one allele, which severely compromises aminoacylation. On the other hand, none of the missense mutations in adult-onset leukodystrophy patients were in the editing domain, whereas at least one missense mutation was in the aminoacylation domain, leading to relatively partial reduction in aminoacylation activity. The location of the compound heterozygous mutations in the present case is consistent with the theory.
In conclusion, we describe a Japanese woman with novel compound heterozygous mutations in AARS2, the first report of leukodystrophy caused by AARS2 mutations in Asia. This is also the subsequent report after the first description in 2014 for six adult-onset leukodystrophy patients by AARS2 mutations. All reported cases including the present case are apparently sporadic without family history and caused by compound heterozygous mutations, making the correct diagnosis very difficult. Therefore, if you will notice the characteristic MRI findings, and especially in female, the existence of ovarian failure, you should examine the AARS2 gene.
Further identification of disease-causing mutations with detailed clinical descriptions including MRI and menstrual history will provide diagnostic clues, and will contribute to unraveling the disease mechanism, and hopefully lead to the development of treatment strategies.
References
Kaye, E. M. & Moser, H. Where has all the white matter gone? Unraveling the mysteries of leukoencephalopathies. Neurology 62, 1464–1465 (2004).
Renaud, D. L. Inherited leukoencephalopathies. Semin. Neurol. 32, 3–8 (2012).
Schiffmann, R. & van der Knaap, M. S. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 72, 750–759 (2009).
Tsurusaki, Y., Koshimizu, E., Ohashi, H., Phadke, S., Kou, I., Shiina, M. et al. De novo SOX11 mutations cause Coffin-Siris syndrome. Nat. Commun. 5, 4011 (2014).
Rademakers, R., Baker, M., Nicholson, A. M., Rutherford, N. J., Finch, N., Soto-Ortolaza, A. et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 44, 200–205 (2012).
Nicholson, A. M., Baker, M. C., Finch, N. A., Rutherford, N. J., Wider, C., Graff-Radford, N. R. et al. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology 80, 1033–1040 (2013).
Konno, T., Tada, M., Koyama, A., Nozaki, H., Harigaya, Y., Nishimiya, J. et al. Haploinsufficiency of CSF-1 R and clinicopathologic characterization in patients with HDLS. Neurology 82, 139–148 (2014).
van der Knaap, M. S., Kamphorst, W., Barth, P. G., Kraaijeveld, C. L., Gut, E. & Valk, J. Phenotypic variation in leukoencephalopathy with vanishing white matter. Neurology 51, 540–547 (1998).
Fogli, A., Gauthier-Barichard, F., Schiffmann, R., Vanderhoof, V. H., Bakalov, V. K., Nelson, L. M. et al. Screening for known mutations in EIF2B genes in a large panel of patients with premature ovarian failure. BMC Womens Health 4, 8 (2004).
Mathis, S., Scheper, G. C., Baumann, N., Petit, E., Gil, R., van der Knaap, M. S. et al. The ovarioleukodystrophy. Clin. Neurol. Neurosurg. 110, 1035–1037 (2008).
Bugiani, M., Boor, I., Powers, J. M., Scheper, G. C. & van der Knaap, M. S. Leukoencephalopathy with vanishing white matter: a review. J. Neuropathol. Exp. Neurol. 69, 987–996 (2010).
Dallabona, C., Diodato, D., Kevelam, S. H., Haack, T. B., Wong, L. J., Salomons, G. S. et al. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology 82, 2063–2071 (2014).
Gotz, A., Tyynismaa, H., Euro, L., Ellonen, P., Hyotylainen, T., Ojala, T. et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 88, 635–642 (2011).
Taylor, R. W., Pyle, A., Griffin, H., Blakely, E. L., Duff, J., He, L. et al. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA 312, 68–77 (2014).
Calvo, S. E., Compton, A. G., Hershman, S. G., Lim, S. C., Lieber, D. S., Tucker, E. J. et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 4, 118ra10 (2012).
Euro, L., Konovalova, S., Asin-Cayuela, J., Tulinius, M., Griffin, H., Horvath, R. et al. Structural modeling of tissue-specific mitochondrial alanyl-tRNA synthetase (AARS2) defects predicts differential effects on aminoacylation. Front. Genet. 6, 21 (2015).
Acknowledgements
This study was supported by a Grant-in-Aid of Health Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan (TY, HY and NM). This study was also supported by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research (A) and (C)); the fund for the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Japan Science and Technology Agency; a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Takeda Science Foundation; and the Strategic Research Program for Brain Science from Japan Agency for Medical Research and Development (NM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Hamatani, M., Jingami, N., Tsurusaki, Y. et al. The first Japanese case of leukodystrophy with ovarian failure arising from novel compound heterozygous AARS2 mutations. J Hum Genet 61, 899–902 (2016). https://doi.org/10.1038/jhg.2016.64
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.64
This article is cited by
-
A Review of Brain and Pituitary Gland MRI Findings in Patients with Ataxia and Hypogonadism
The Cerebellum (2023)
-
Deficiency of the mitochondrial ribosomal subunit, MRPL50, causes autosomal recessive syndromic premature ovarian insufficiency
Human Genetics (2023)
-
AARS2-Related Leukodystrophy: a Case Report and Literature Review
The Cerebellum (2022)
-
Whole-exome sequencing reveals new potential genes and variants in patients with premature ovarian insufficiency
Journal of Assisted Reproduction and Genetics (2022)
-
A novel EIF4ENIF1 mutation associated with a diminished ovarian reserve and premature ovarian insufficiency identified by whole-exome sequencing
Journal of Ovarian Research (2019)