Abstract
The focus of this review is to evaluate the influence of β2-adrenergic receptor (ADRB2) polymorphism on human physiological function and in turn on athletic performance. A narrative review is conducted on available literature using MedLine, Pubmed and the Cochrane Library to document the location and function of ADRB2 receptors, and specifically to address the influence of genetic polymorphisms on cardiovascular, respiratory, metabolic and musculoskeletal systems and athletic performance. Search terms included ADRB2, endurance and polymorphism. Previous literature exploring the genetic composition of athletes has proposed that alterations in the genetic structure result in an enhancement in their capacity to achieve successful aerobic phenotypes such as a higher VO2max and increased fat oxidation. Polymorphism of the Gly16Glu27 haplotype is believed to promote positive aerobic phenotypes and regulate optimal lipolysis. Greater knowledge of the ADRB2 polymorphism can aid in understanding the specific phenotypes that are altered, which may influence performance. Until the interaction between fatigue and athletic performance is better understood, the development of appropriate training principles to enhance genetically polymorphic aerobic phenotypes remains complicated. Following the review, there is still no distinctive evidence for the predictive ability of the polymorphism of ADRB2 genotype for the purpose of identifying potential elite athletes.
Similar content being viewed by others
Introduction
Endurance performance can be defined as the ability to exert the highest possible performance, power and velocity for a period of time beyond 20 min.1 Endurance performance demands precise maintenance of optimal cardiovascular control of parameters such as heart rate, stroke volume, cardiac output and mean arterial blood pressure. Elite endurance athletes, such as distance runners, road cyclists and triathletes, demonstrate extraordinary feats of prolonged physiological functioning because of enhancement in aerobic phenotype. Central and peripheral physiological attributes such as increased left ventricular contractility, an increased proportion of type I muscle fibers with greater mitochondrial and capillary density reflecting higher VO2max, higher lactate threshold, increased fat oxidation capacity and greater control toward maintaining acid-base homeostasis, separate these athletes from other sports people and the general population.2, 3, 4
The Human Genome Project5 states that a 0.1% variation separates the DNA of any two given individuals. This 0.1% variation is due to single-nucleotide polymorphisms as a result of epigenetic factors influencing the nucleic acid. Polymorphism can be defined simply as natural variations within the nucleic acid that express a unique trait. Indeed, genetic polymorphisms have been associated with a number of disease manifestations such as coronary heart disease and hypertension.6
Training-induced increases in muscle mitochondrial density promote efficient oxygen and substrate utilization by working muscles, leading to the delay in the onset of muscle fatigue due to substrate depletion. Although these adaptations can be enhanced through long-term training preparations, responses elicited from individuals differ even if it is derived from similar training regimes, thus highlighting the influence of genetic variation on performance.
Studies exploring the β2-adrenergic receptor (ADRB2) have highlighted the importance of this gene in endurance performance because of its expression throughout the cardiovascular, respiratory, metabolic and musculoskeletal systems, along with its influence on lipid metabolism to regulate energy expenditure from adipose tissue.7 Understanding the influence of ADRB2 receptors on various systems around the body has potential applications for endurance athletes, as it has been shown to enhance aerobic phenotype. Phenotypes such as an increase in stroke volume and cardiac output are due to the interaction between catecholamines and the vast number of β-receptors expressed throughout the body. Catecholamine regulation is important as it has a central role in energy expenditure.8
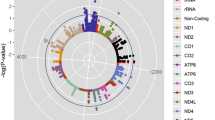
The cytogenetic location of the ADRB2 gene is on Chromosome 5, between bands q31 and q32.9 The most distinctive polymorphisms that have been identified in the gene encoding the ADRB2 receptor protein include Arg16Gly and Gln27Glu10 (Figure 1).11 These polymorphisms are associated with increases in lipolytic sensitivity (Arg16Gly and Gln27Glu variants), as well as with expressing unique properties in relation to binding and signal transduction, such as regulating vascular tone.8, 12
The cytogenetic location of ADRB2 receptors and localization of single-nucleotide polymorphisms (SNPs).11
The abnormal function of the β-receptor is related to numerous diseases affecting the heart, lungs and vessels, such as heart failure, asthma and hypertension.13 This implies that knowledge of the specific phenotypes that are altered because of single-nucleotide polymorphism allows for understanding the abnormal function of the β-receptor, and thus determines the physiological effects during physical exertion.
The focus of this review is to determine the potential influence of single-nucleotide polymorphisms of the ADRB2 gene on endurance performance in athletes through the examination of receptor function on cardiovascular, respiratory, metabolic and musculoskeletal systems, as well as epigenetics and its interaction with the environment to develop potential implications in tailoring training regimens in individuals with genetic polymorphisms.
The β2-adrenergic receptors
β-receptors can be found throughout the body, facilitating a sympathetic nervous system response of ‘fight-or-flight.’ β-receptors can be further divided into β1, β2 and β3, with the β2-adrenergic receptors belonging to the G-protein-coupled receptor super family that is primarily found presynaptically.14 The receptor consists of an extracellular amino terminal, seven transmembrane domains and an intracellular carboxyl terminal.15 The stimulation of catecholamine functions through Gαs receptors to cause a conformational change in the receptor and thus stimulates adenylate cyclase to increase intracellular cAMP. The second messenger system leads to the stimulation of protein kinase A, which phosphorylates endoplasmic reticulum proteins to promote the opening of ligand-gated Ca2+ channels to facilitate muscular contraction through myosin phosphorylase.14
Stimulation of β2-receptors promotes relaxation of smooth muscle in the gastrointestinal tract, and also aids in bronchodilation; it promotes gluconeogenesis and glycogenolysis in the liver and skeletal muscles, as well as insulin secretion; in the cardiovascular system, β2-receptor stimulation increases heart rate, stroke volume, cardiac output and arterial dilation and increases renin secretion from the kidney, thus having a role in regulating blood pressure14, 16 (Figure 2). In addition, ADRB2 receptors are also found in fat cells, in which they actively engage in lipolysis.17 Stimulation of ADRB2 receptors in adipocytes is due to increased sympathetic activity and catecholamine release.18
The inhibition of β-receptors has been shown to decrease the cardiovascular drift (increased oxygen uptake with fat contributing as the main fuel source) during prolonged exercise, possibly because of the influence of ADRB2 on lipolysis.19 In addition, β2-receptors are prone to desensitization; however, studies have suggested that differences in phenotype, such as increased receptor expression, are likely to promote superior cardiovascular performance.20, 21, 22 Thus, it can be seen that alteration of the gene responsible for these actions can influence phenotypical characteristics and potentially lead to enhanced aerobic capacity and performance.
The genetic influence of adrenergic-β2 receptors
Currently, there are potentially two major physiological mechanisms proposed that influence the genetic composition of ADRB2 receptors. Wolfarth et al.7 propose that, first, catecholamines have a crucial role in the regulation of substrate mobilization and thus influence body mass and composition. The same study also indicated that homozygous Arg16 allele subjects could benefit favorably from catecholamine stimulation resulting in a lower body weight because of the regulation of fat mobilization by ADRB2 receptors,23 and in addition from an optimal weight-to-strength ratio as a consequence of achieving a lean body mass, allowing for efficient mobilization of substrates to maximize endurance performance. Second, the polymorphisms of the cardiovascular phenotypes such as increased myocardial inotropy, chronotropy and cardiac output, as well as enhanced gluconeogenesis, bronchodilation and vasodilatation, are likely to improve cardiovascular function and manifest in a higher VO2 peak because of the vast distribution of β-adrenoceptors expressed through the body.7, 14 Exploring each system is necessary to understand the impact of polymorphism of ADRB2 on endurance performance.
Musculoskeletal system
The contribution of genetic variation toward skeletal muscle fiber-type proportions has been estimated between 40 and 50%.24 Consequently, understanding the influence of genetic polymorphism on the musculoskeletal system is essential.
Endurance athletes have the ability to delay the onset of fatigue by promoting increased oxygen and substrate delivery to working muscles. The main by-products of oxidative metabolism from endurance exercises are the accumulation of hydrogen ions (lactic acid–acidosis) and heat (hyperthermia).25 An increase in mitochondrial and capillary density and myoglobin content in type I muscle fibers increases the aerobic efficiency of muscle fibers and promotes glycogenolysis in the muscle from substrates in the liver and adipose tissue, allowing the athlete to continue to exert force for a longer duration.14, 26, 27
ADRB2 receptors have an important and significant role in skeletal muscle function. Furthermore, it is known that 99% of muscle adrenergic receptors are β2-receptors.28 The enhanced signaling of cAMP from the ADRB2 gene influences the glucose pathway through the activation of glycogen phosphorylase and the inhibition of glycogen synthase. Research conducted on mutant hyperactive glycogen synthase male mice showed an inverse relationship between the level of expression of β2-receptors and muscle glycogen content.29 This suggests that the increased levels of cAMP decrease fructose-2, 6-bisphosphate, and thus suppress glycolysis.29 Endurance athletes may benefit from such a phenotype as glucose content is spared, thus delaying the onset of fatigue through the prolonged use of fat as the main energy source.
Epinephrine stimulates β2-receptors to promote lipolysis,30, 31 which encourages glycerol to enter the glycolysis pathway and thus generate pyruvate and, consequently, lactate.32 The lactate entering the Cori cycle functions to synthesize glucose to preserve blood glucose homeostasis.27 This increase in lactate also increases the activity of the Na+K+-ATPase pump and thus the conversion of muscle glycogen to lactate—promoting the protective mechanism of the body to use lactate as a form of energy rather than glucose oxidation.32 As a result, epinephrine stimulation promotes high rates of aerobic glycolysis, allowing organs such as the heart and the brain to maintain specific functions that require a high rate of cytoplasmic ATP.33, 34
Further investigations on pathological conditions affecting skeletal muscles have illustrated the importance of the ADRB2 gene in the regulation of skeletal muscle function. A study by Xu et al.35 observed a significant disequilibrium between ADRB2 polymorphisms at amino-acid positions 16 and 27, and a greater incidence of Myasthenia Gravis in homozygous Arg16 patients. This is because of increased ADRB2 receptor antibodies lowering nicotinic-receptor expression and thus disturbing the immune response to the disease. This blocking of nicotinic receptors prevents the binding of acetylcholine at the neuromuscular junction and leads to a loss of muscle force.14 In contrast, homozygous Glu27 patients do not fully express the mature form of the receptor and are thus less likely to induce antibody formation.35 Consequently, there is increased nicotinic-receptor expression, thus permitting the binding of acetylcholine to nicotinic receptors at the neuromuscular junction.36
Endurance athletes may benefit from such a phenotype as the neurochemical processes of muscle contraction remain unhindered, lowering the susceptibility of nicotinic-receptor blockage, allowing more binding sites to become available, thus increasing muscle force. As the majority of these studies have been based on the clinical population, further research on the athletic population is necessary to determine the influence of homozygous Glu27 alleles on the physiology of skeletal muscle contractility to allow a better understanding of the impact that this variation has on prolonging muscle force during endurance activities.
Cardiovascular system
The impact of ADRB2 receptors on cardiovascular function is large. Approximately 30% of β2-adrenoceptors are found in the atria of the heart, allowing for calcium influx to stimulate ventricular contraction.37 Homozygous Gly16 alleles were found to influence the cardiovascular function of a nonathletic, healthy population during rest, light and heavy exercise by demonstrating greater stroke volume, cardiac output and mean arterial pressure, in addition to the enhanced left ventricular ejection fraction.38, 39 This implies that polymorphism of the Arg16Gly haplotype in ADRB2 receptors enhances the cardiovascular function during exercise. This is potentially significant for endurance athletes requiring greater cardiac output during high-intensity activity.
It has been proposed that the difference between individuals expressing homozygous Arg16 and Gly16 alleles is due to an association observed between the ADRB2 receptor density per lymphocyte, which is related to the degree of β-adrenergic receptors located on the myocardial tissue, and stroke volume among homozygous Gly16 allele carriers.20 An impact on exercise performance has been seen in healthy subjects expressing the homozygous Gly16 allele, demonstrating an increase in exercise capacity up to 2 h.40 Furthermore, exposure to β-agonists delayed the downregulation of β-receptors in subjects with Glu27 alleles.41 Although the exact mechanism is unknown, it is likely that the response of increased receptor density to an agonist is not linear because of a reduction in active receptor signaling after agonist-induced desensitization.42, 43
ADRB2 also influences vascular function through the promotion of vasodilatation from β2-receptors located in the vascular endothelium of blood vessels.14 A particular study showed that normotensive Gly16 allele subjects expressed lower vascular resistance at rest following isometric handgrip exercises at 40% of maximal contraction.44 Furthermore, numerous studies have illustrated that normotensive subjects expressing homozygous Arg16 alleles showed a greater potential for desensitization in venous circulation as a result of isoproterenol infusion in forearm blood flow, along with attenuated blood flow, when compared with homozygous Gly16 alleles.22, 45
Another potential suggestion is the impact of polymorphism of ADRB2 receptors in the cardiovascular system in response to sympathomimetic stimulus during exercise until fatigue. As subjects become tired, central command in the brainstem causes vagal withdrawal, resulting in increased epinephrine levels, thus increasing the heart rate and cardiac output.46 This suggests that perhaps myocardial β-receptors are stimulated in response to cardiac sympathetic nerve activity, leading to increased chronotropy and inotropy.47
On the basis of these findings, Dishy et al. proposed that the optimal haplotype combination for cardiovascular response during short-term exercise is Gly16Glu27. This is because of increased receptor numbers, resistance to desensitization and enhanced stroke volume and cardiac output.22 Furthermore, the Gly16Glu27 haplotype combination may be used as a marker for talent identification of athletes at an early age.
Respiratory system
ADRB2 receptors located primarily in the lungs are essential for the maintenance of bronchial smooth muscle homeostasis. Spanning from the trachea to the alveoli, β2-receptors aid in the maintenance of gas exchange by regulating airway tone, to promote bronchodilation during exercise and enhance ventilation with minimal airway resistance.48 Some studies have observed that genetic polymorphisms only influence bronchodilation during heavy exercise, and not at rest or recovery.20, 48
Previous studies have explored the influence of vagal withdrawal and catecholamine release in the regulation of airway tone. The study by Snyder et al.48 observed minimal increases in forced expiratory flow at 50% of the forced vital capacity (FEF50) during light exercise in healthy subjects expressing homozygous Gly16 alleles. As this occurred at a time when catecholamine levels were unchanged, vagal withdrawal is most likely to have influenced exercise-induced broncodilation in these subjects.49
The influence of variations in ADRB2 during long-duration exercise demonstrated that Gly16 allele individuals sustained bronchodilation for a longer duration when compared with Arg16 allele individuals.20 The onset of bronchoconstriction is likely to be observed because of bronchial hyperresponsiveness as a result of receptor downregulation.50 Furthermore, healthy homozygous Arg16 subjects illustrated a sharp decline below baseline levels in their maximal expiratory flow rates during recovery, whereas Gly16 subjects consistently maintained FEF50 levels above baseline—suggesting that the Gly16 allele is more resistant to alveolar-vascular desensitization48 (that is Gly16 allele subjects were able to oxygenate blood more efficiently from the alveolar-capillary gas exchange, thus permitting FEF50 levels to be maintained above baseline during recovery). This may be explained by the increased receptor reserve that is found in airways. Receptor reserve is characterized by the amount of additional receptors available for stimulation and signaling, as adjacent receptors become desensitized.51 Liang and Mills51 found that receptor density in the lungs is ∼18 2fmol mg−1, compared with 85 fmol mg−1 found in the heart. This difference allows for increased receptor sites for agonist stimulation, thus promoting enhanced gas exchange. Endurance athletes will benefit from enhanced gas exchange, as it improves diffusion of oxygen to working muscles while removing carbon dioxide from circulation.
It was also found that Arg16 subjects have greater lung fluid accumulation, along with a reduced diffusing capacity for carbon monoxide.52, 53 A reduced alveolar-capillary exchange thus causes a reduction in oxygen delivery. Therefore, endurance athletes will not benefit from such a phenotype as it disrupts the delivery of substrates to working muscles and leads to hypoxia and damage to cells. A reduced simulation of epithelial sodium channels in the lungs is seen in Arg16 subjects, which lessens fluid removal capacity from the lungs and consequently affects pulmonary function during prolonged exercise.20
Metabolic system
Training-induced increases in muscle mitochondrial density promote oxygen and substrate delivery to working muscles. Mobilization of adipose tissue triglycerides provides the largest fuel source during endurance exercise. β-receptors facilitate lipolysis during exercise through the stimulation of sympathetic nervous system activity, which counteracts circulating insulin action on adipocytes during prolonged exercise.18, 54
Stimulation of the sympathetic nervous system through the onset of exercise promotes the release of catecholamines—epinephrine and norepinephrine. The β2-receptor subtype is more effective under epinephrine exposure.14 Savard et al.30 and Wahrenberg et al.31 observed a 20–35% increase in lipolysis in response to catecholamines after 30 and 90 min of aerobic exercise in healthy subjects, respectively. This is supported by the findings of Crampes et al.,55 in which endurance-trained athletes showed increased epinephrine-stimulated lipolysis in adipocytes.
The Arg16Gly polymorphism has been shown to affect lipolysis because of the sensitivity of β2-receptors in adipose tissue. Specifically, obese homozygous Gly16 and Glu27 subjects reported a 50% enlargement in adipocytes and a fivefold increase in lipolytic sensitivity to agonists.8 Although additional investigations are required to combine the knowledge of ADRB2 receptors and their impact on the metabolic system during endurance exercises, it can, however, be hypothesized that endurance athletes expressing the Gly16Glu27 haplotype are likely to benefit from enhanced lipolysis, thus allowing for the improvement in aerobic phenotypes.
There is still inadequate evidence to confidently outline the polymorphic impact of ADRB2 receptors on cardiovascular, respiratory, metabolic and musculoskeletal systems on aerobic phenotypes. A greater understanding of specific phenotypes that are altered because of single-nucleotide polymorphism and receptor interaction with substrates and catecholamines will further enhance our knowledge of their influence to benefit athletic performance.
The influence of the environment on potential trainability of endurance phenotypes expressed in polymorphic ADRB2 receptors
To fully understand athletic performance, it is necessary to consider the interaction between the genetic composition of an athlete and their environment. The notion of ‘nature vs nurture’ can greatly influence the development and performance of an athlete.55
Epigenetics, or external factors that alter the phenotype without affecting the genotype, are an important consideration when exploring gene–environment interactions.56 Homogeneous genotypes, as found among monozygotic twins, are altered by epigenetic factors that affect each tissue independently, thus modifying the unique expression and function of the gene. Factors such as smoking habits, diet and level of physical activity are likely to influence the division and differentiation of cells, resulting in phenotype modifications.57, 58 Hawley59 expanded on this notion by stating that chronic adaptations to skeletal muscle physiology are likely to be due to the cumulative effects of external factors that have influenced the aerobic phenotype. As a result, geographical locations, altitude and training regimes heavily influence the development of the athlete.
By understanding the gene–environment interaction, it allows for the facilitation of a model for training regimes on the basis of genotype. Aspects such as intensity and volume of training can aid in the enhancement of genetic polymorphisms to further augment the physiological enrichment and performances during endurance activities. This can also promote the usage of genotyping as a means for talent identification.
Training protocols aim to increase the availability of oxygen and substrates and promote efficient energy production of working muscles.60 Popular techniques to improve fatigue resistance and increase endurance have been a form of interval training—consisting of numerous exercise bouts, incorporated with short rest intervals of activity at a lower intensity.61 The objective is to improve lactate kinetics, stimulate neurological muscle fiber recruitment patterns and enhance fatigue resistance and athletic performance.62, 63, 64, 65
As noted previously by Savard et al.,30 Wahrenberg et al.31 and Levy et al.,32 epinephrine stimulation of β2-receptors increases the activity of the Na+K+-ATPase enzyme. Evidence shows that endurance athletes have an increased concentration of the Na+K+-ATPase enzyme in the plasma membrane.66 A possible explanation is the activation of Na+K+-ATPase enzyme by catecholamines, thus stimulating action potentials and force production in skeletal muscles.67 It is thus possible that training regimes can take advantage of the force production capacity of skeletal muscles through the implementation of high-intensity sprint training exercises to increase Na+K+-ATPase concentration and thus increase the amount of force produced by muscle fibers on stimulation.68
The polymorphism of subjects expressing homozygous Gly16 alleles demonstrated greater potential for trainability because of higher aerobic performances and increased responses to exercises.38 Snyder et al.20 also proposed that the Gly16Glu27 haplotype represents a more positive response to exercise. On the contrary, studies have shown that the homozygosity of the Glu27 allele and the Glu27Glu haplotypes in healthy subjects can lead to significantly lower levels of oxygen consumption and thus a reduction in VO2max.23 Thus, it is more likely that healthy subjects expressing the homozygous Gly16 alleles have the greatest potential for the trainability of endurance performance, as it is highly expressed in the cardiovascular, respiratory and metabolic systems.8, 38, 39, 49 However, conflicting evidence exists to suggest that homozygous Gly16 allele carriers are negatively associated with endurance performance.7
Hypoxic training is also considered as an effective way to increase endurance capacity,69 as ADRB2 receptors are highly expressed in the lungs and blood vessels. Conducting aerobic exercises at high altitude produces a hypoxic environment, leading to increased hematocrit and blood viscosity.70 Consequently, there is an increased secretion of erythropoietin, which increases myoglobin content and thus increases oxygen-carrying capacity.71 This increase in oxygen delivery is because of a smaller difference in the alveolar-arterial oxygen partial pressure, suggesting enhanced pulmonary gas exchange in response to hypoxia.72 Furthermore, at high altitudes, ADRB2 receptors may be involved in increased oxygen delivery during acclimatization.73
There is a scarcity of literature exploring the physiological responses of highly trained athletes to a modified training program. As endurance athletes already exhibit superior aerobic performance, modification of training protocols to promote high-intensity training has been suggested.74 A study by Hickson et al.75 illustrated that the implementation of high-intensity interval-style training can improve aerobic performance. The study consisted of running or cycling for 6 days per week at 100% of VO2max for 6 × 5 mins with a 2-min break in between bouts. The findings showed that there were increases in VO2max and time to exhaustion. Numerous other studies76, 77, 78 have also illustrated the advantage of high-intensity interval-style training as a necessity to further challenge endurance athletes to obtain training benefits.
There is a further lack of evidence to provide optimal intensity and volume of training, as most elite athletes are unwilling to experiment with their training programs.79 Acknowledging the influence of environmental factors on the alteration of phenotype can establish a direction toward developing potential training regimes. The protocol adopted by numerous studies to examine the influence of genetic polymorphisms follows a similar intensity of between 75 and 80% of VO2max, conducted 2–3 times a week.21, 60, 79 Consequently, further investigations are necessary to optimize the intensity and volume of training on the basis of the ADRB2 genotype. This may be achieved by examining the effects of manipulation of training variables, such as high volumes vs high-intensity training. Further investigations into tailoring training protocols to specific genotypes should also prove beneficial. Although there are currently very limited studies in this field, the introduction of high-intensity sprint exercises to homozygous Glu27 allele subjects may improve force production capacity, or reducing rest periods between high-intensity interval training in homozygous Gly16 allele subjects could improve respiratory function, as it is shown that they are able to maintain FEF50 levels above baseline during recovery. These speculations need to be addressed scientifically in the future to allow tailored training regimes to maximize the athletic performance of ADRB2 polymorphic subjects.
It is important to note that no single aspect, whether it is genetic or environmental, can influence the phenotype of an athlete. Rather, understanding the influence of epigenetic factors on phenotypes can provide an insight into the interaction of genes and environment on athletic performance.80, 81 Furthermore, until a greater understanding of the interaction between fatigue and athletic performance is determined, developing appropriate training practices to enhance aerobic phenotypes remains complicated.82
Conclusion
In conclusion, the polymorphisms of ADRB2 receptors can positively or negatively influence the cardiovascular, respiratory, metabolic and musculoskeletal systems to enhance endurance phenotypes because of the vast expression of β-adrenoceptors throughout the body. The Gly16Glu27 haplotype seems to elicit a more positive response toward exercise. Future investigations should aim to gather information toward understanding the impact of ADRB2 receptors, particularly on metabolic and musculoskeletal systems, in addition to gaining greater insight into the implications of pathological studies on athletic performance.
Further knowledge of the gene–environment interaction and specific epigenetic factors can aid in the appreciation of the demographics and population that are more likely to enrich the gene polymorphism. This information can aid in the development of training protocols and talent identification criteria that take into account the specific demands of the sport. As the Gly16Glu27 haplotype combination has been shown to be optimal for short-term exercise, future studies should direct their attention toward developing training protocols to further optimize this phenotype. Antipodal reports indicate the use of genotyping for the purpose of optimizing training and talent identification. Future studies need to explore this notion to provide evidence for any correlation. However, there is still no distinctive evidence for the predictive value of these variants for the purpose of identifying potential elite athletes.83
References
Hickson, R. C. Interference of strength development by simultaneously training for strength and endurance. Eur. J. Appl. Physiol. Occup. Physiol. 45, 255–263 (1980).
Coyle, E. F., Martin, W. H. III, Sinacore, D. R., Joyner, M. J. & Holloszy, J. O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. 57, 1857–1864 (1984).
Robergs, R. A., Ghiasvand, F. & Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R502–R516 (2004).
Coyle, E. F., Sidossis, L. S., Horowitz, J. F. & Beltz, J. D. Cycling efficiency is related to the percentage of type I muscle fibres. Med. Sci. Sports Exerc. 24, 782–788 (1992).
The Human Genome Project. The Science Behind the Human Genome Project published online 12 June 2009 〈http://www.ornl.gov/sci/techresources/Human_Genome/project/info.shtml〉 (2009).
Gambano, G., Anglani, F. & D’Angelo, A. Association studies of genetic polymorphisms and complex disease. Lancet. 355, 308–311 (2000).
Wolfarth, B., Rankinen, T., Mühlbaur, S., Scherr, J., Boulay, M. R., Pérusse, L. et al. Association between a β2-adrenergic receptor polymorphism and elite endurance performance. Metab. Clin. Exp. 56, 1649–1651 (2007).
Large, V., Hellström, L., Reynisdottir, S., Lönnqvist, F., Eriksson, P., Lannfelt, L. et al. Human β2-adrenergic gene polymorphisms are highly frequent in obesity and associate with altered adipocyte β2-adrenoceptor function. J. Clin. Invest. 100, 3005–3013 (1997).
Bray, M. S., Hagberg, J. M., Pérusse, L., Rankinen, T., Roth, S. M., Wolfarth, B. et al. The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Med. Sci. Sports Exerc. 41, 34–72 (2009).
Green, S. A., Cole, C., Jacinto, M., Innis, M. & Liggett, S. B. A polymorphism of the human β2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J. Biol. Chem. 268, 23116–23121 (1993).
Pharmacogenomics Knowledge Base (PharmGKB). Find Data by Type: Genes: ADRB2–Variants published online 14 October 2009 〈http://www.pharmgkb.org/do/serve?objId=PA39&objCls=Gene#tabview=tab2〉 (2009).
Barnes, M. R. Bioinformatics for Genetics: A Bioinformatics Primer for the Analysis of Genetic Data 2nd edn (John Wiley & Sons, West Sussex,, 2007).
Williams, A. G., Dhamrait, S. S., Wootton, P. T. E., Day, S. H., Hawe, E., Payne, J. R. et al. Bradykinin receptor gene variant and human physical performance. J. Appl. Physiol. 96, 938–942 (2004).
Rang, H., Dale, M., Ritter, J. & Moore, P. Pharmacology 6th edn. (Churchill Livingstone, Edinburgh, 2007).
Garland, E. M. & Biaggoni, I. Genetic polymorphisms of adrenergic receptors. Clin. Auton. Res. 11, 67–78 (2001).
Fitzpatrick, D., Purves, D. & Augustine, G. Neuroscience 3rd edn (Sinauer, Massachusetts, 2004).
Hoffstedt, J., Iliadou, A., Pedersen, N. L., Schalling, M. & Arner, P. The effect of beta2 adrenoceptor gene Thr164Ile polymorphism on human adipose tissue lipolytic function. Br. J. Pharmacol. 133, 708–712 (2001).
Kjaer, M., Christensen, N. J., Sonne, B., Richter, E. A. & Galbo, H. Effect of exercise on epinephrine turnover in trained and untrained male subjects. J. Appl. Physiol. 59, 1061–1067 (1985).
Eriksson, P., Dahlman, I., Ryden, M., Hoffstedt, J. & Arner, P. Relationship between beta2 adrenoceptor gene haplotypes and adipocyte lipolysis in women. Int. J. Obes. Relat. Metab. Disord. 28, 185–190 (2004).
Snyder, E. M., Johnson, B. D. & Joyner, M. J. Genetics of β2-adrenergic receptors and the cardiopulmonary response to exercise. Exerc. Sports Sci. Rev. 36, 98–105 (2008).
Snyder, E. M., Beck, K. C., Dietz, N. M., Eisenach, J. H., Joyner, M. J., Turner, S. T. et al. Agr16Gly polymorphism of the β2-adrenergic receptor is associated with differences in cardiovascular function at rest and during exercise in humans. J. Physiol. 571, 121–130 (2006).
Dishy, V., Sofowora, G. G., Xie, H. G., Kim, R. B., Byrne, D. W., Stein, C. M. et al. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N. Engl. J. Med. 345, 1030–1035 (2001).
Moore, G. E., Shuldiner, A. R., Zmuda, J. M., Ferrell, R. E., McCole, S. D. & Hagberg, J. M. Obesity gene variant and elite endurance performance. Metabolism 50, 1391–1392 (2001).
Simoneau, J. A. & Bouchard, C. Genetic determinism of fiber type proportion in human skeletal muscle. J. FASEB 9, 1091–1095 (1995).
Coyle, E. F. Physiological determinants of endurance exercise performance. J. Sci. Med. Sport 2, 181–189 (1999).
Germann, W. J. & Stanfield, C. L. Principles of Human Physiology 2nd edn. (Benjamin Cummings, San Francisco, 2005).
McArdle, W. D., Katch, F. I. & Katch, V. L. Exercise Physiology: Energy, Nutrition and Human Performance 5th edn (Lippincott, Williams & Wilkins, Baltimore, 2001).
Liggett, S. B., Shah, S. D. & Cryer, P. E. Characterization of beta-adrenergic receptors of human skeletal muscle obtained by needle biopsy. Am. J. Physiol. Endocrinol. Metab. 254, E795–E798 (1988).
Parker, G. E., Pederson, B. A., Obayashi, M., Schroeder, J. M., Harris, R. A. & Roach, P. J. Gene expression profiling of mice with genetically modified muscle glycogen content. Biochem. J. 395, 137–145 (2006).
Savard, R., Despres, J. P., Marcotte, M., Theriault, G., Tremblay, A. & Bouchard, C. Acute effects of endurance exercise on human adipose tissue metabolism. Metabolism 36, 480–485 (1987).
Wahrenberg, H., Engfeldt, P., Bolinder, J. & Arner, P. Acute adaptation in adrenergic control of lipolysis during physical exercise in humans. Am. J. Physiol. Endocrinol. Metab. 253, E383–E390 (1987).
Levy, B., Desebbe, O., Montemont, C. & Gibot, S. Increased aerobic glycolysis through β2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 30, 417–421 (2008).
Schurr, A., Payne, R. S., Miller, J. J. & Rigor, B. M. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J. Neurochem. 69, 423–426 (1997).
Kline, J. A., Thornton, L. R., Lopaschuk, G. D., Barbee, R. W. & Watts, J. A. Lactate improves cardiac efficiency after hemorrhagic shock. Shock 14, 215–221 (2000).
Xu, B. Y., Haung, D., Pirskanen, R. & Lefvert, A. K. β2-adrenergic receptor gene polymorphism in myasthenia gravis. Clin. Exp. Immunol. 119, 156–160 (2000).
Green, S., Turki, J., Innis, M. & Liggett, S. B. Amino-terminal polymorphisms of the human β2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry 33, 9414–9419 (1994).
Brodde, O. E. Beta-1 and beta-2 adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol. Rev. 43, 203–242 (1991).
Tang, W., Devereux, R. B., Kitzman, D. W., Province, M. A., Leppert, M., Oberman, A. et al. The Arg16Gly polymorphism of the β2-adrenergic receptor and left ventricular systolic function. Am. J. Hypertens. 16, 945–951 (2003).
Snyder, E. M., Hulsebus, M. L., Turner, S. T., Joyner, M. J. & Johnson, B. D. Genotype related differences in β2 adrenergic receptor density and cardiac function. Med. Sci. Sports Exerc. 38, 882–886 (2006).
Frey, M. J., Mancini, D., Fischberg, D., Wilson, J. R. & Molinoff, P. B. Effect of exercise duration on density and coupling of beta-adrenergic receptors on human mononuclear cells. J. Appl. Physiol. 66, 1494–1500 (1989).
Bruck, H., Leineweber, K., Beilfuss, A., Weber, M., Heusch, G., Philipp, T. et al. Genotype-dependent time course of lymphocyte beta2-adrenergic receptor down-regulation. Clin. Pharmacol. Ther. 74, 255–263 (2003).
Chong, L. K. & Peachell, P. T. Beta-adrenoceptor reserve in human lung: a comparison between airway smooth muscle and mast cells. Eur. J. Pharmacol. 378, 115–122 (1999).
Drury, D. E., Chong, L. K., Ghahramani, P. & Peachell, P. T. Influence of receptor reserve on beta-adrenoceptor-mediated responses in human lung mast cells. Br. J. Pharmacol. 124, 711–718 (1998).
Eisenach, J. H., Barnes, S. A., Pike, T. L., Sokolnicki, L. A., Masuki, S., Dietz, N. M. et al. Arg16/Gly β2-adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J. Appl. Physiol. 99, 1776–1781 (2005).
Garovic, V. D., Joyner, M. J., Dietz, N. M., Boerwinkle, E. & Turner, S. T. Beta2-adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J. Physiol. 546 (Part 2), 583–589 (2003).
Rowell, L. B. Human Cardiovascular Control (Oxford University Press, New York,, 1993).
Martin, C. E., Shaver, J. A., Leon, D. F., Thompson, M. E., Reddy, P. S. & Leonard, J. J. Autonomic mechanisms in hemodynamic responses to isometric exercise. J. Clin. Invest. 54, 104–115 (1974).
Snyder, E. M., Beck, K. C., Dietz, N. M., Joyner, M. J., Turner, S. T. & Johnson, B. D. Influence of β2-adrenergic receptor genotype on airway function during exercise in healthy adults. Chest 129, 762–770 (2006).
Squires, R. W. Exercise training after cardiac transplantation. Med. Sci. Sports Exerc. 23, 686–694 (1991).
D’Amato, M., Vitiani, L. R., Petrelli, G., Ferrigno, L., di Pietro, A., Trezza, R. et al. Association of persistent bronchial hyperresponsiveness with β2-adrenoceptor (ADRB2) haplotypes. Am. J. Respir. Crit. Care Med. 158, 1968–1973 (1998).
Liang, W. & Mills, S. E. Quantitative analysis of beta-adrenergic receptor subtypes in pig tissues. J. Anim. Sci. 80, 963–970 (2002).
Snyder, E. M., Beck, K. C., Turner, S. T., Hoffman, E. A., Joyner, M. J. & Johnson, B. D. Genetic variation of the beta2 adrenergic receptor is associated with differences in lung fluid accumulation in humans. J. Appl. Physiol. 102, 2172–2178 (2007).
Snyder, E. M., Turner, S. T. & Johnson, B. D. Beta2-adrenergic receptor genotype and pulmonary function in patients with heart failure. Chest 130, 1527–1534 (2006).
Mendenhall, L. A., Swanson, S. C., Habash, D. L. & Coggan, A. R. Ten days of exercise training reduces glucose production and utilization during moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 266, EI36–EI43 (1994).
Crampes, F., Beauville, M., Riviere, D. & Garriques, M. Effect of physical training in humans on the response of isolated fat cells to epinephrine. J. Appl. Physiol. 61, 25–29 (1986).
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. (suppl.) 33, 245–254 (2003).
Cooney, C. A. Are somatic cells inherently deficient in methylation metabolism? A proposed mechanism for DNA methylation loss, senescence and aging. Growth Dev. Aging 57, 261–273 (1993).
Bennett-Baker, P. E., Wilkowski, J. & Burke, D. T. Age-associated activation of epigenetically repressed genes in the mouse. Genetics 165, 2055–2062 (2003).
Hawley, J. A. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin. Exp. Pharmacol. Physiol. 29, 218–222 (2002).
Rankinen, T., Pérusse, L., Borecki, I., Chagnon, Y. C., Gagnon, J., Leon, A. S. et al. The Na+-K+-ATPase α2 gene and trainability of cardiorespiratory endurance: the HERITAGE Family Study. J. Appl. Physiol. 88, 346–351 (2000).
Daniels, J. & Scardina, N. Interval training and performance. J. Sports Med. 1, 327–334 (1984).
Tanaka, K. & Matsuura, Y. Marathon performance, anaerobic threshold and onset of blood lactate accumulation. J. Appl. Physiol. 57, 640–643 (1984).
LeMond, G. & Gordis, K. Greg LeMond's Complete Book of Bicycling (Perigree Books, New York,, 1990).
Coetzer, P., Noakes, T. D., Sanders, B., Lambert, M. I., Bosch, A. N., Wiggins, T. et al. Superior fatigue resistance of elite black South African distance runners. J. Appl. Physiol. 75, 1822–1827 (1993).
Hawley, J. A. State of the art training guidelines for endurance performance. S. Afr. J. Sports Med. 2, 7–12 (1995).
Evertsen, F., Medbø, J. I., Jebens, E. & Nicolaysen, K. Hard training for 5 months increases Na+-K+ pump concentration in skeletal muscle of cross-country skiers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 272, R1417–R1424 (1997).
Overgaard, K., Nielsen, O. B. & Clausen, T. Effects of reduced electrochemical Na+ gradient on contractility in skeletal muscle: role of the Na+-K+ pump. Eur. J. Physiol.—Pflügers Arch. 434, 457–465 (1997).
McKenna, M. J., Schmidt, T. A., Hargreaves, M., Cameron, L., Skinner, S. L. & Kjeldsen, K. Sprint training increases human skeletal muscle Na+-K+-ATPase concentration and improves K+ regulation. J. Appl. Physiol. 75, 173–180 (1993).
Roels, B., Millet, G. P., Marcoux, C. J. L., Coste, O., Bentley, D. J. & Candau, R. B. Effects of hypoxic interval training on cycling performance. Med. Sci. Sports Exerc. 37, 138–146 (2005).
Stobdan, T., Karar, J. & Qadar Pasha, M. A. High altitude adaptation: genetic perspectives. High Alt. Med. Biol. 9, 140–147 (2008).
Ge, R. L., Witkowski, S., Zhang, Y., Alfrey, C., Sivieri, M., Karlsen, T. et al. Determinants of erythropoietin release in response to short-term hypobaric hypoxia. J. Appl. Physiol. 92, 2361–2367 (2002).
Dempsey, J. A., Reddan, W. G., Birnbaum, M. L., Forster, H. V., Thoden, J. S., Grover, R. F. et al. Effects of acute through life-long hypoxic exposure on exercise pulmonary gas exchange. Respir. Physiol. 13, 62–89 (1971).
Mazzeo, R. S. & Reeves, J. T. Adrenergic contribution during acclimatization to high altitude: perspectives from pikes peak. Exerc. Sport Sci. Rev. 31, 13–18 (2003).
Laursen, P. B. & Jenkins, D. G. The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. J. Sports Med. 32, 53–73 (2002).
Hickson, R. C., Bomze, H. A. & Holloszy, J. O. Linear increase in aerobic power induced by a strenuous program of endurance exercise. J. Appl. Physiol. 42, 372–376 (1977).
Green, H., Tupling, R., Roy, B., O’Toole, D., Burnett, M. & Grant, S. Adaptations in skeletal muscle exercise metabolism to a sustained session of heavy intermittent exercise. Am. J. Physiol. Endocrinol. Metab. 278, E118–E126 (2000).
Keith, S. P., Jacobs, I. & McLellan, T. M. Adaptations to training at the individual anaerobic threshold. Eur. J. Appl. Physiol. 65, 316–323 (1992).
Burke, J., Thayer, R. & Belcamino, M. Comparison of effects of two interval-training programmes on lactate and ventilatory thresholds. Br. J. Sports. Med. 28, 18–21 (1994).
Hawley, J. A., Myburgh, K. H., Noakes, T. D. & Dennis, S. C. Training techniques to improve fatigue resistance and enhance endurance performance. J. Sports Sci. 15, 325–333 (1997).
Davids, K. & Baker, J. Genes, environment and sports performance: why the nature-nurture dualism is no longer relevant. J. Sports Med. 37, 961–980 (2007).
Brutsaert, T. D. & Parra, E. J. What makes a champion? explaining variation in human athletic performance. Respir. Physiol. Neurobiol. 151, 109–123 (2006).
Noakes, T. D. Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand. J. Med. Sci. Sports 10, 123–145 (2000).
MacArthur, D. G. & North, K. N. Genes and human elite athletic performance. Hum. Genet. 116, 331–339 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarpeshkar, V., Bentley, D. Adrenergic-β2 receptor polymorphism and athletic performance. J Hum Genet 55, 479–485 (2010). https://doi.org/10.1038/jhg.2010.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2010.42
Keywords
This article is cited by
-
Complexity of genetics in the athlete phenotype: A commentary on Adrenergic-β2 receptor polymorphism and athletic performance
Journal of Human Genetics (2010)