Abstract
There has been great interest in the development of extremely potent antibacterial lantibiotic peptides for medicinal use and in food preservation. Of central importance to this endeavor is a strong understanding of which parts of the peptides are required for activity and which parts are expendable. Nisin, lacticin 481, nukacin ISK-1, mersacidin, lacticin 3147 and haloduracin represent many different types of lantibiotic peptides. In recent years, considerable advances toward understanding the structure–activity relationship of each of these lantibiotic systems have been achieved. This review will focus on the individual systems and the valuable information obtained from the many mutants produced.
Similar content being viewed by others
Introduction
Many antibiotics found on the market today are derived from natural sources. Microbes (fungi, bacteria and actinomycetes) provide a wealth of structures with promising activities, and modification of these natural leads has resulted in many classes of clinical drugs.1 For example, the bacterially derived tetracycline antibiotic family contains both naturally occurring structures (for example, tetracycline) and semisynthetic compounds (for example, doxycycline). Erythromycin was isolated from Saccharopolyspora erythraea, and clarithromycin is a synthetic analog produced to improve the acid stability of the compound.2
Lantibiotics
Lantibiotics are a family of antimicrobial peptides produced by gram-positive bacteria to target other bacteria. The producing organism is immune to the activity of its own peptides, the mechanisms by which this occurs have been reviewed.3 ABC transporters and dedicated immunity proteins are frequently involved. Lantibiotics are synthesized by the ribosome with an N-terminal leader sequence and then they are heavily posttranslationally modified.4 The leader sequence is believed to keep the peptides inactive while inside the producing bacterial cell and to act as a handle for recognition by modification enzymes.5 Many of these peptides are extremely potent antibacterial agents with minimum inhibitory concentrations in the nM range.6 Lantibiotics are active against several very common food spoilage organisms (for example, Listeria monocytogenes and Clostridium botulinum) and show very promising activity against resistant Staphylococcus aureus and enterococcal infections.7 Many lantibiotics originate from lactic acid bacteria, which are classified as ‘generally recognized as safe’. As such, these bacteria are considered non-threatening to human health; therefore, their lantibiotic products are very appealing choices for food safety and clinical applications.
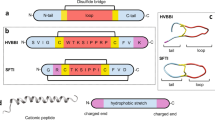
Lantibiotics are composed of the unusual amino acids, lanthionine (lan) and methyllanthionine (melan), hence the name lan-containing antibiotics. In addition, lantibiotics contain many other unusual amino acids, such as dehydrated amino acids, D-amino acids, α-ketoamides and S-aminovinylcysteine (see Figure 1). These novel functionalities are introduced from a linear precursor peptide by the action of several modification enzymes.6 Hydroxy amino acids are converted to dehydroamino acids and then lan and melan can be formed by Michael addition of a cysteine thiol. Aminovinylcysteine residues are formed by the addition of a vinyl cysteine (derived from a C-terminal cysteine) onto a dehydroamino acid. D-alanine results from a stereoselective reduction of dehydroalanine. The α-ketoamides are formed following cleavage of the leader sequence, which exposes an N-terminal-dehydrated residue that tautomerises to an imine that is then hydrolyzed to give the α-ketoamide. The structures of lantibiotics are non-linear as a result of the cyclic thioether bridges of the lan, melan and aminovinylcysteine residues.
For classification purposes, individually active lantibiotic peptides are generally divided into two groups, A and B, based upon the overall shape of the peptide.4 Group A contains peptides that are elongated, whereas group B contains peptides that are more compact and globular. Group A is further subdivided into AI and AII, wherein the lan and melan of the peptides in AI are produced by two modification enzymes (LanB and LanC), whereas the lan and melan of the peptides in AII are produced by a single modification enzyme (LanM). A third group of lantibiotics has come to the forefront of peptide research in the last 10 years. Two-component lantibiotics consist of two lantibiotic peptides that are produced by the same organism and display high levels of synergistic activity. For many of the two-peptide systems, each peptide shows structural and sequence similarities to individually active lantibiotics. α-Peptides resemble the globular peptides of group B and β-peptides resemble the more elongated peptides in group A.8
Much research attention has focused on the utilization of lantibiotics in food preservation/safety. However, these peptides also show promise for broader use in the field of human/animal therapeutics.7 Despite the long-term use of lantibiotics in the food industry (for example, nisin has been used for more than 40 years), very little bacterial resistance has developed to these peptides. With growing concerns in the scientific and medical communities over increasing levels of antibiotic resistance, lantibiotic peptides could provide a promising alternative to conventional small molecule antibiotics. It is believed that one of the reasons that bacteria are not developing resistance to lantibiotics is the manner in which the peptides exert their activity. Many lantibiotics act in several different ways to kill bacteria. The peptides can bind to lipid II and interfere with the biosynthesis of the bacterial cell wall, resulting in the inhibition of bacterial growth. They can also form pores in the cell membrane, resulting in the loss of cellular contents and death of the cell. It is believed that this dual mode of action makes it much more challenging for bacteria to develop functional resistance against lantibiotic peptides.6 Two-component lantibiotics may exert only one of these modes of action (binding to lipid II) when administered independently and both modes of action when used together (lipid II binding and pore formation).9
There are still fundamental challenges to the successful utilization of many lantibiotics as therapeutic agents. Thus far, their use has been limited to topical applications, as many of these peptides are prone to degradation by enzymes present in the gastrointestinal tract. Additionally, many lantibiotics are not stable over the full pH range, as under basic conditions, many peptides undergo oxidation of the lan/melan thioethers, resulting in a loss of antimicrobial activity.10 These structural disadvantages along with a desire for smaller/simpler peptides have driven much of the research into lantibiotic analogs. If clinically useful peptides are to be developed from lantibiotics, solutions need to be found for these inherent challenges.7
This review will focus on recent work investigating the structure–activity relationships (SARs) of lantibiotic peptides. Specifically, nisin, lacticin 481, nukacin ISK-1, mersacidin, haloduracin and lacticin 3147 will be discussed. Within the last 5 years, several alternative methods for studying the SAR of lantibiotic peptides have been utilized in conjunction with the traditional approach of mutation studies.
Nisin
Nisin, produced by Lactococcus lactis, is often referred to as the ‘prototypical’ lantibiotic7 and is a type A1 lantibiotic. There are several natural nisin variants, the two most common being nisin A (res 27-His) and nisin Z (res27-Asn). These peptides contain one lan and four melan rings, along with three dehydrated amino acids. Discovered in 1928, nisin is active against many gram-positive strains, including important food spoilage bacteria, such as Listeria monocytogenes and Clostridium botulinum.6 Nisin also shows considerable promise as a human therapeutic, especially for the treatment of drug-resistant bacterial infections.7 As nisin SAR studies have been extensively reviewed,4, 6, 7, 11, 12 we have chosen to limit this discussion to several recent studies.
A series of single, double and triple mutants at the A and B rings of nisin A were produced by mutagenesis (see Figure 2).13 A large number of the mutants of ring A retained activity and indeed two, double mutant I4K/L6I and triple mutant I4K/S5F/L6I, displayed increased potency against indicator organisms. The introduction of a positive charge into ring A of nisin appears to have a positive effect on the activity of the peptide, and it would be interesting to see how other charged amino acids at different N-terminal positions affect activity. In fact, several other related lantibiotics (nisin U and epidermin) contain a lysine at position 4. Of the mutants produced for ring A, only three were without an aromatic residue. This is particularly interesting, as the native peptide does not possess an aromatic residue in this ring. It would therefore seem that ring A is quite flexible to amino-acid substitution and further investigations could be very fruitful.
Ring B of nisin was found to be far less amenable to amino-acid substitution. One analog was found that retained natural activity and it consisted of the rather conservative Gly10Thr mutation. It was reported that ring B was very sensitive to the size of amino acids, with bulky residues giving poor or no activity. This lowered activity is in part because the cysteine thiol at position 11 was sometimes unable to cyclize onto the dehydrobutyrine (Dhb) at position 8. The interruption of ring B likely caused the abolition of activity in these cases. Several truncated peptides were also produced in which residues 23–34 were removed. In most cases, a considerable loss in activity was observed; however, when natural nisin and the triple mutant I4K/S5F/L6I were truncated, they were found to be only 10-fold less active than natural nisin. Mode of action studies indicated that the peptides did not form pores and, thus, were likely to work solely by interference with lipid II and cell wall biosynthesis.13
A recent study focused on the hinge region of nisin A.14 Random mutagenesis yielded three mutants, N20P, M21V and K22T that each possessed activity greater than natural nisin A. Encouragingly, the M21V mutant showed activity against Listeria monocytogenes making it a potential food preservative, whereas N20P was active against S. aureus and K22T was active against Streptococcus agalactiae, indicating they could be used in veterinary medicine to treat mastitis. All three mutants also displayed improved activity against methicillin-resistant S. aureus. Despite the existence of a large library of hinge mutants, the relationship between the activity and the nature of the amino acids in the hinge region is still somewhat unpredictable. However, a few trends have emerged. Aromatic residues along with negatively charged residues are very detrimental to activity; by comparison, small, polar, hydrophobic or positively charged amino acids can produce active peptides. Also, the hinge must remain flexible to allow the two halves of nisin to move with respect to each other.
These studies add to the great wealth of information about nisin and encouragingly boast the identification of several mutants with increased activity.
Lacticin 481
Lacticin 481, a class AII lantibiotic produced by Lactococcus lactis,15, 16 contains one melan and two lan rings in addition to one Dhb residue. LctM, the biosynthetic enzyme responsible for the production of the dehydrated and cyclic portions of lacticin 481, has been purified and utilized in vitro for protein engineering.17
The importance of individual amino acids to the biological activity of lacticin 481 has been investigated using several in vitro and in vivo techniques (see Figure 3). The N-terminal Lys1 was removed by proteolytic cleavage and the truncated lacticin 481 retained activity.17 When the processing enzyme LctT (believed to cleave off the leader sequence to produce lacticin 481) is not present, production of a small quantity of a truncated lacticin 481 missing the first five residues of the N terminus is observed. This peptide is found to possess activity, albeit at a 10-fold decrease.18 Ring A of lacticin 481 is conserved in several other lantibiotics (for example, mersacidin, lacticin 3147 A1 and haloduracin-α) and thus the amino acids are generally believed to be very important to activity. The mutant Glu18Ala was produced by in vitro processing of a linear precursor peptide with reconstituted LctM.19 Very surprisingly, this mutation did not abolish activity, unlike with the equivalent mersacidin and haloduracin-α mutants that were both strongly affected by this change.8, 20, 21, 22 Other mutants in ring A were found to retain activity, suggesting there is some degree of flexibility in the amino acids of this ring.23 Mutagenesis has been used to produce weakly active analogs containing the changes S4T, H8P, Q20T and truncation at S27.24, 25
Recent lacticin 481 mutants and structure–activity relationship conclusions. (a) Structure of lacticin 481 with active mutants shown above the structure and inactive mutants shown below the structure. Portions of the peptide vital to activity are shown bordered with a dotted line. (b) Structures of the unnatural amino acids included in the mutasynthesis.
The importance of the ring structures in lacticin 481 has also been studied.23 It appears that each ring can exist as either lan or melan and the peptide remains active. However, anytime a ring was opened, the peptide lost all antimicrobial activity (ring B, rings A and B, and rings B and C). It is important to note that in all of the ring opening analogs, ring B was disturbed. Therefore, it is hard to know whether ring A or C is truly vital, or if the result is purely due to loss of ring B. It would seem that any interruption of ring B of this peptide would drastically alter the overall shape of the molecule, and this may be a leading cause of the loss of activity. The opening of ring B would allow the peptide to become a far less globular/more elongated and flexible molecule. Peptide analogs were produced, incorporating unnatural amino acids via a combination of chemical peptide synthesis and in vitro enzymatic synthesis. A number of non-proteinogenic amino acids were substituted into the structure of lacticin 481 along with several common amino-acid substitutions. Several of the unnatural analogs maintained a high level of activity; these contained conservative substitutions of aromatic amino acids (Trp19Nal, Phe21Pal and Phe23hPhe) and were all located in the C ring of the peptide.26 Overall lacticin 481 seems to be quite tolerant to amino-acid substitutions, so long as they do not result in a global structural change.
Nukacin ISK-1
Nukacin ISK-1 was isolated from S. warneri ISK-1 and contains one melan as well as two lan rings and a Dhb.27, 28 Nukacin ISK-1 is another member of the AII family of lantibiotics, differing from lacticin 481 by only 7 of the 27 residues and most of these are small changes, for example, aspartic acid to glutamic acid. Indeed the members of the AII group have several regions that are highly conserved across all the peptides. It has been postulated that these residues must be conserved for a reason and are likely needed for the peptides to exert antibacterial activity.29
An initial study looked at the effect of the overall charge of nukacin ISK-1 on its antibacterial activity (and its ability to bind to an anionic membrane). The N terminus of this peptide contains three cationic lysine residues (giving nukacin ISK-1 an overall plus-three charge) that were postulated to be vital for interaction with the cell membrane and thus the antibacterial activity.30 Mutants were produced that abolished the overall charge of nukacin ISK-1 both by deletion of the first three lysine residues and by substitution of all the three with alanine residues (see Figure 4). These changes resulted in a large decrease in activity compared with wild-type nukacin ISK-1. Additionally, neither analog was able to bind to a model anionic membrane. Thus, these positively charged residues are necessary for membrane binding and biological activity.
A subsequent study produced around 100 different mutants of nukacin-ISK-1 covering every position in the peptide. In this work, each of the three lysines (1, 2 and 3) was altered independently and it was found that the loss of a single lysine was tolerated.29 The authors postulate that this finding suggests the positive charges of the lysine amino acids are non-essential for the antimicrobial activity of nukacin ISK-1. We find this assertion somewhat unlikely, as from the data, one can see that the loss of one charged lysine does not abolish the activity of nukacin ISK-1; however, the loss of all three lysine residues does. It would seem, therefore, that a small amount of charge flexibility exists, but an overall positive charge is required for binding to the membrane, it is also likely needed for activity. Indeed in their earlier study, the authors discussed an analog with two further lysine residues added to nukacin ISK-1 that showed no increase in antibacterial activity, suggesting that the magnitude of charge may be less important so long as an overall positive charge exists.30
The first analogs of the AII group to achieve enhanced activity when compared with the wild-type peptide were produced for nukacin ISK-1. Asp13Glu and Val22Ile were both found to display specific activities more potent than nukacin ISK-1 itself. It is noteworthy that both of these mutations are very conservative and in the case of Asp13, this is the only mutant at this position that displays any activity and is thus effectively a vital amino acid for activity. Val10Leu, His15Ser, His15Phe, Phe21Trp and Thr24Ile were found to possess equivalent or inferior activity profiles to natural nukacin ISK-1.
From this study, it would appear that except for a few residues within the ring structures, most of the variability lies in the N-terminal tail of the peptide (46 partially active mutants for only 8 residues versus 36 partially active mutants for 19 residues). Additionally, five of these residues can be mutated to non-polar, polar or charged residues with retention of activity. By comparison, only His15 and Ser27 in the cyclic portion of the peptide show such flexibility. Although there appears to be more tolerance to change in the N terminus, the two analogs displaying increased activity are both mutants of amino acids in the cyclic end of the peptide.
Mersacidin
Mersacidin is a small, globular lantibiotic that is produced by Bacillus HIL Y-85,54728.31 This class B peptide comprises three melan rings along with a dehydroalanine and an aminovinylmethylcysteine residue. Mersacidin has shown very promising activity against methicillin-resistant S. aureus.32, 33 Mersacidin interacts with a different part of lipid II compared with the current drug of last resort, vancomycin, suggesting it could be a complementary treatment strategy.34
The investigations of SAR for mersacidin have been undertaken using a mutagenesis approach (see Figure 5). An initial limited study produced the mutants F3L, S16I and E17A, all of which were found to be inactive or very weakly active.21
Recent mersacidin mutants and structure–activity relationship conclusions. Structure of mersacidin with active mutants shown above the structure and inactive mutants shown below the structure. Position of amino-acid insertions denoted by arrow. Portions of the peptide vital to activity are shown bordered with a dotted line.
A subsequent systematic production of mersacidin analogs via mutagenesis yielded a far larger number of mutants.22 Three libraries were designed in which amino acids were substituted, inserted or deleted. None of the shortened peptides (amino-acid deletions) were produced by this system, suggesting that contraction of the rings produces substrates that cannot be processed by the mersacidin biosynthetic enzymes. Only the second ring of mersacidin would tolerate insertion of amino acids and these variants showed almost complete abolition of activity. Conservative amino-acid substitutions were relatively well tolerated throughout the molecule and gave many active mutants. The second ring of mersacidin showed the most flexibility to changes in amino acids, with several non-conservative changes (G7N, G8Q, G9H, G10Y and P6H) retaining activity. Leu5, Glu17 and Ile19 appear to be vital for the activity of the peptide, as any changes at these locations completely abolished activity. The authors note that it is unsurprising that fewer changes are tolerated in the third ring, as there is considerable conservation of amino acids in this ring among related peptides (such as lacticin 481, lacticin 3147 A1 and haloduracin-α). Several mutants (F3W, V11I, L14I and S16Dhb) were found that displayed equivalent or better activity than mersacidin. Several double mutants were designed based on these results (F3W-L14I and V11I-L14I), and although they retained activity, they did not show any further gains over the single mutants. To test whether the lan rings would retain activity in the place of melan, attempts were made to install serine at positions 2, 4, 13 and 15; however, no peptide products were found for these mutations. It would appear that the biosynthetic machinery is unable to produce the peptide with lan in place of melan. This result leaves the importance of the melan ring unknown.22
Although most of the mutations that retained some degree of activity were found in the second ring of mersacidin, those that showed the greatest activity were spread throughout the molecule. This suggests that although the ring B is a good place to focus further analog studies, it should by no means be to the exclusion of the rest of the molecule. It would also be very useful to obtain more information about the importance of the ring structures of mersacidin to its antibacterial activity.
Lacticin 3147
The most studied of the two-component lantibiotics is lacticin 3147. Isolated from Lactococcus lactis,35 lacticin 3147 consists of a compact A1 peptide and a flexible, elongated A2 peptide. Lacticin 3147 A1 has two lan and two melan rings along with a D-alanine and two Dhb residues. Lacticin 3147 A2 comprises one lan and two melan rings, two D-alanines, two Dhb residues and an α-ketoamide capped N terminus.36 Lacticin 3147 has potential for use in the clinic to treat drug-resistant organisms, such as methicillin-resistant S. aureus, and is also currently being investigated for use in veterinary medicine to treat bacterial mastitis in dairy cows.7, 37 Like nisin, lacticin 3147 has many possibilities for application in food preservation.38
The large number of lacticin 3147 analogs have yielded some very important information about the functionality vital for the individual activity of these peptides and also the functionality that results in the synergism of the two peptides.
Mutagenesis investigations of the unusual D-alanine amino acids in both peptides found a lacticin 3147 A1 mutant that was 4-fold less active when dehydroalanine was present and 2-fold less active when Dhb (coded by threonine) or glycine was present (see Figure 6). Interestingly, when either L-alanine or L-valine was introduced, the desired mutant was not isolated. For lacticin 3147 A2, no peptide was isolated when dehydroalanine replaced D-alanine; however, when L-alanine was introduced, single mutants were 4-fold less active and a double mutant was 16-fold less active. A single mutant S9G retained the activity of the wild type, whereas a double mutant was 4-fold less active. A single mutant S9Dhb was half as active as the wild type, whereas a double mutant was 8-fold less active. A single mutant S9V was 16-fold less active than the wild type, whereas a double mutant was not detected.39
Recent lacticin 3147 mutants and structure–activity relationship conclusions. Structures of lacticin 3147 with active mutants shown above the structures and inactive mutants shown below the structures. Double mutations represented by a dotted line. Portions of the peptides vital to activity are shown bordered with a dotted line.
A very comprehensive analysis via alanine scanning was undertaken for both A1 and A2 peptides. Mutants that disturbed the ring structures of either peptide were found to be inactive or were not processed by the biosynthetic enzymes. Of the other peptides produced, none were more active than the natural peptides, and overall, it was found that the A2 peptide (particularly it's N terminus) was more tolerant to change than A1.40 A further mutagenesis study was undertaken to probe for mutants that decreased the activity of the natural peptides. More mutants were detected for A1 (as it is less flexible to amino-acid alteration) than A2. The inactive mutants were associated with amino acids forming the lan and melan rings in both peptides as well as amino acids within the B, C and D rings of A1.41
Staphylococcin C55 is a two-component peptide that shares considerable sequence identity with lacticin 3147. Several amino acids in lacticin 3147 A1 were mutated to mimic those in staphylococcin C55α in order to investigate whether activity was retained. N15K and A17N both displayed good activity, whereas the L21A mutation did not result in the production of any peptide. The authors propose that this is because of incompatibility with the processing machinery.42 The authors were also able to demonstrate that lacticin 3147 A1-processing enzymes could produce fully functional staphylococcin C55α, so it is rather strange that they will not tolerate the L21A substitution.
The importance of the composition of the lan and melan residues in lacticin 3147 A2 has been studied using chemical synthesis, as it allows access to peptides that would never be created or processed by the enzymatic machinery that is relied upon for in vivo analog production. Three different ring analogs of lacticin 3147 A2 were synthesized using solid phase peptide synthesis and were then compared with natural A2. Perhaps the most obvious change was to produce an analog containing three lan rings.43 This peptide was found to have no individual activity; however, intriguingly it still displayed synergistic activity with natural lacticin A1. The observed synergistic activity of lan–lacticin 3147 A2 with natural lacticin 3147 A1 was 100-fold less potent than for the natural peptide. This result suggests that the Me groups of lacticin 3147 A2 are required for direct complexation of the peptide with lipid II and thus the independent activity of the peptide is abolished when they are absent. It would seem that the Me groups are not vital to the formation of an interaction between the lacticin 3147 A2 and the lipid II-lacticin 3147 A1 complex.
The second analog was designed to overcome some of the issues with stability that plague lan-containing peptides, namely that when the thioether of lan or melan is oxidized, the peptide loses its antibacterial activity. This is problematic as the oxidation can occur very readily in the presence of molecular oxygen in air. An analog of lacticin 3147 A2, with oxygen atoms in place of the sulfur atoms in the rings, was designed with the aim of maintaining antibacterial activity while simultaneously improving the oxidative stability of the peptide. Using a similar methodology to the lan analog, oxa-lacticin 3147 A2 was chemically synthesized and tested for antibacterial activity.44 The peptide maintained intrinsic activity (albeit 20-fold less than native lacticin 3147 A2), but the synergism of activity with natural A1 was completely lost. This result is the complete opposite of that seen for lan-lacticin 3147 A2 and suggests that the heteroatom of lan and melan is very important for an interaction between lipid II-lacticin 3147 A1 and lacticin 3147 A2.
A further ring analog was prepared in which the heteroatom of lan and melan is replaced with an olefin. As well as testing the importance of having a heteroatom present in the ring (for hydrogen bonding and so on), this analog also represents a ring-expanded structure. The synthesis of olefin–lacticin 3147 A2 was accomplished using solid phase peptide synthesis techniques combined with ring-closing metathesis to cyclize the rings.45 Antibacterial testing found that the peptide analog was not active either independently or in combination with natural lacticin 3147 A1. It would appear that the increase in the size of the rings has a powerful negative effect on the activity of the peptide. It could also be that the absence of a heteroatom in the ring damages activity.
Two very different approaches to study the SARs of lacticin 3147 A1 and A2 have been taken. On one hand, Ross, and coworkers have utilized a mutagenesis approach to produce a large number of single amino-acid mutants to examine the importance of each amino acid in the two-component peptide system.39, 40, 41, 42 On the other hand, Vederas and coworkers have used chemical synthesis to investigate the importance of the lan and melan rings with a view towards producing simpler and more stable analogs.43, 44, 45
Haloduracin
Haloduracin is a two-component lantibiotic isolated from Bacillus halodurans consisting of a globular α-peptide resembling the class B lantibiotics and an elongated β-peptide similar to class A.46, 47 Haloduracin-α has one cysteine, one lan and two melan rings. Haloduracin-β contains one lan and three melan rings as well as three Dhbs. These two peptides have been found to exert synergistic antibacterial activity as well as individual activity. Haloduracin is produced under basic conditions and is considerably more stable at pH 7 than nisin (produced under acidic conditions). With a similar spectrum of activity to nisin, haloduracin could be extremely useful in clinical applications.8
Each of the rings in both haloduracin peptides has been systematically opened, by substitution of the cysteine involved in ring formation with an alanine residue (see Figure 7). These analogs were accessed by in vitro conversion of linear precursors with reconstituted HalM1 or HalM2. The N-terminal cysteine of haloduracin-α is not needed for activity.20 Ring A is important but not essential for activity, as a large reduction in activity is observed. Ring B is not essential for activity and the mutant retains strong activity. This is unexpected as several other lantibiotics (mersacidin, lacticin 481 and lacticin 3147 A1) possess a similar ring structure and it was believed to be vital to activity. Additionally, Glu22Ala and Glu22Gln mutants show little and no activity, respectively, which is consistent with what was observed for mersacidin. Therefore, it would seem that the conserved sequence seen in ring B is very substrate specific and in each different peptide the activity is related to its exact placement in the molecule. It seems that it is not simple to predict whether its individual amino acids are required for activity.
Ring C is very important to the antimicrobial functioning of haloduracin-α, as loss of this ring causes activity to be greatly diminished. A Ser26Ala mutant was also produced and was found to retain activity.8, 20, 46
Ring A of haloduracin-β was found to have a small effect on activity, with a moderate reduction of activity in the mutant. Rings C and D were both shown to be important, as mutations resulted in very little remaining activity. Production of the mutant of ring B was not successful, as it appeared multiple rings were affected, thus the absence of activity for this mutant does not provide any real information as to whether ring B is vital to activity. Ser22Ala and Dhb18Ala mutants were produced and both displayed good activity, suggesting neither is important for activity.
Haloduracin is a very new lantibiotic and already we know a considerable amount about its important structural features. It will be very interesting to learn more about its scope for individual amino acids and how much the structure can be simplified.
Conclusion
Over the last 20–30 years, the scientific community has been intrigued by how lantibiotics can exert such powerful antibacterial activity. What exactly about their structures contributes to their activity? It can be no accident that these peptides contain such high levels of posttranslational modification. Additionally, some lantibiotics have been found that have remarkably similar sequences and these natural analogs strongly imply that there is a degree of flexibility to the structure/function relationship that leaves room for the artificial production of novel lantibiotics.
In the past, genetic manipulation has been utilized to produce variants of lantibiotics that contain simple amino-acid replacements. This approach allows for the importance of a single amino acid to be investigated. Although an incredibly useful tool (and the source of by far the most SAR data to date), there are several drawbacks to this system. Firstly, analogs are limited to natural amino-acid-containing peptides. Secondly, because of the extensive posttranslational modifications of the peptides, the natural producer organism must be used for the production of mutants to allow for the requisite modification enzymes. Thirdly, if mutant peptides with an altered spectrum of activity are produced, they may get past the immunity mechanisms of their producing organism. This would result in the death of the producing bacteria and would stop any further synthesis of this potentially very active peptide. In this way, peptides with improved activity and specificity could go undetected. Finally and most importantly, as the production of desired analogs relies upon the modification machinery accepting altered substrates, many analogs are not produced and so cannot be tested for activity. In the last 5 years, other methods for producing variants of lantibiotics have been used. At the opposite end of the spectrum, total chemical synthesis has provided access to peptides analogs with complete chemical control and no amino-acid limitations. In the middle, sits in vitro ‘mutasynthesis’ that enables the introduction of unnatural amino acids in a linear peptide and then subsequent use of enzymes to increase complexity (such as the introduction of rings). Thus ‘mutasynthesis’ appears to offer the advantages of both synthesis and mutagenesis.
With so many varied tools for producing lantibiotic peptides, our understanding of their structure activity–relationships continues to grow and develop, leading towards the development of antimicrobial peptides for the future.
References
Li, J. W.- H. & Vederas, J. C. Drug discovery and natural products: end of an era or an endless frontier? Science 325, 161–165 (2009).
Fernandes, P. B. et al. In vitro and in vivo evaluation of A-56268 (TE-031): a new macrolide. Antimicrob. Agents Chemother. 30, 865–873 (1986).
Draper, L. A., Ross, R. P., Hill, C. & Cotter, P. D. Lantibiotic immunity. Curr. Protein Peptide Sci. 9, 39–49 (2008).
Bierbaum, G. & Sahl, H.- G. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 10, 2–18 (2009).
Willey, J. M. & van der Donk, W. A. Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 61, 477–501 (2007).
Chatterjee, C., Paul, M., Xie, L. & van der Donk, W. A. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–683 (2005).
Cotter, P. D., Hill, C. & Ross, R. P. Bacterial lantibiotics: strategies to improve therapeutic potential. Curr. Protein Peptide Sci. 6, 61–75 (2005).
Oman, T. J. & van der Donk, W. A. Insights into the mode of action of the two-component lantibiotic haloduracin. ACS Chem. Biol. 4, 865–874 (2009).
Wiedemann, I. et al. The mode of action of the lantibiotic lacticin 3147- a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61, 285–296 (2006).
Wilson-Stanford, S. et al. Oxidation of lanthionines renders the lantibiotic nisin inactive. Appl. Environ. Microbiol. 75, 1381–1387 (2009).
Asaduzzaman, S. M. & Sonomoto, K. Lantibiotics: diverse activities and unique modes of action. J. Biosci. Bioeng. 107, 475–487 (2009).
Lubelski, J., Rink, R., Khusainov, R., Moll, G. N. & Kuipers, O. P. Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell Mol. Life Sci. 65, 455–476 (2008).
Rink, R. et al. Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl. Environ. Microbiol. 73, 5809–5816 (2007).
Field, D., O’Connor, P. M., Cotter, P. D., Hill, C. & Ross, R. P. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol. Microbiol. 69, 218–230 (2008).
Rince, A. et al. Cloning, expression and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 60, 1652–1657 (1994).
Piard, J.- C., Muriana, P. M., Desmazeaud, M. J. & Klaenhammer, T. R. Purification and partial characterization of lacticin 481, a lanthionine-containing bacteriocin produced by Lactococcus lactis subsp. lactis CNRZ 481. Appl. Environ. Microbiol. 58, 279–284 (1992).
Xie, L. et al. Lacticin 481: In vitro reconstitution of lantibiotic synthetase activity. Science 303, 679–681 (2004).
Uguen, P. et al. Maturation by LctT is required for biosynthesis of full-length lantibiotic lacticin 481. Appl. Environ. Microbiol. 71, 562–565 (2005).
Patton, G. C. & van der Donk, W. A. New developments in lantibiotic biosynthesis and mode of action. Curr. Opin. Microbiol. 8, 543–551 (2005).
Cooper, L. E., McClerren, A. L., Chary, A. & van der Donk, W. A. Structure-activity relationship studies of the two-component lantibiotic haloduracin. Chem. Biol. 15, 1035–1045 (2008).
Szekat, C., Jack, R. W., Skutlarek, D., Färber, H. & Bierbaum, G. Construction of an expression system for site-directed mutagenesis of the lantibiotic mersacidin. Appl. Environ. Microbiol. 69, 3777–3783 (2003).
Appleyard, A. N. et al. Dissecting structural and functional diversity of the lantibiotic mersacidin. Chem. Biol. 16, 490–498 (2009).
Chatterjee, C., Patton, G. C., Cooper, L., Paul, M. & van der Donk, W. A. Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem. Biol. 13, 1109–1117 (2006).
Dufour, A., Hindré, T., Haras, D. & Le Pennec, J.- P. The biology of lantibiotics from the lacticin 481 group is coming of age. FEMS Microbiol. Rev. 31, 134–167 (2007).
Hindré, T. et al. Bacteriocin detection from whole bacteria by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Appl. Environ. Microbiol. 69, 1051–1058 (2003).
Levengood, M. R., Knerr, P. J., Oman, T. J. & van der Donk, W. A. In vitro mutasynthesis of lantibiotic analogues containing nonproteinogenic amino acids. J. Am. Chem. Soc. 131, 12024–12025 (2009).
Kimura, H., Nagano, R., Matsusaki, H., Sonomoto, K. & Ishizaki, A. A bacteriocin of strain Pediococcus sp. ISK-1 isolated from nukadoko, bed of fermented rice bran. Biosci., Biotechnol., Biochem. 61, 1049–1051 (1997).
Sashihara, T. et al. A novel lantibiotic, ISK-1, of Staphylococcus warneri ISK-1: cloning of the structural gene and identification of the structure. Biosci., Biotechnol., Biochem. 64, 2420–2428 (2000).
Islam, M. R. et al. Evaluation of essential and variable residues of nukacin ISK-1 by NNK scanning. Mol. Microbiol. 72, 1438–1447 (2009).
Asaduzzaman, S. M., Nagao, J.-i., Aso, Y., Nakayama, J. & Sonomoto, K. Lysine-oriented charges trigger the membrane binding and activity of nukacin ISK-1. Appl. Environ. Microbiol. 72, 6012–6017 (2006).
Chatterjee, S. et al. Mersacidin, a new antibiotic from bacillus fermentation, isolation, purification and chemical characterisation. J. Antibiot. 45, 832–838 (1992).
Chatterjee, S. et al. Mersacidin, a new antibiotic from bacillus in vitro and in vivo antibacterial activity. J. Antibiot. 45, 839–845 (1992).
Kruszewska, D. et al. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitic model. J. Antimicrob. Chemother. 54, 648–653 (2004).
Brötz, H., Bierbaum, G., Leopold, K., Reynolds, P. E. & Sahl, H.- G. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 42, 154–160 (1998).
Ryan, M. P., Rea, M. C., Hill, C. & Ross, R. P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62, 612–619 (1996).
Martin, N. I. et al. Structural characterization of lacticin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry 43, 3049–3056 (2004).
Ryan, M. P., Meaney, W. J., Ross, R. P. & Hill, C. Evaluation of lacticin 3147 and a teat seal containing this bacteriocin for inhibition of mastitis pathogens. Appl. Environ. Microbiol. 64, 2287–2290 (1998).
Ross, R. P. et al. Developing applications for lactococcal bacteriocins. Antonie Van Leeuwenhoek 76, 337–346 (1999).
Cotter, P. D. et al. Posttranslational conversion of L-serines to D-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc. Natl Acad. Sci. USA 102, 18584–18589 (2005).
Cotter, P. D. et al. Complete alanine scanning of the two-component lantibiotic lacticin 3147: generating a blueprint for rational drug design. Mol. Microbiol. 62, 735–747 (2006).
Field, D., Collins, B., Cotter, P. D., Hill, C. & Ross, R. P. A system for the random mutagenesis of the two-peptide lantibiotic lacticin 3147: analysis of mutants producing reduced antibacterial activities. J. Mol. Microbiol. Biotechnol. 13, 226–234 (2007).
O’Connor, E. B. et al. Relatedness between the two-component lantibiotics lacticin 3147 and staphylococcin C55 based on structure, genetics and biological activity. BMC Microbiol. 7, 24–38 (2007).
Pattabiraman, V. R., McKinnie, S. M. K. & Vederas, J. C. Solid-supported synthesis and biological evaluation of the lantibiotic peptide Bis (desmethyl) lacticin 3147 A2. Angew. Chem. Int. Ed. 47, 9472–9475 (2008).
Liu, H., Pattabiraman, V. R. & Vederas, J. C. Synthesis and biological activity of oxa-lacticin A2, a lantibiotic analogue with sulfur replaced by oxygen. Org. Lett. 11, 5574–5577 (2009).
Pattabiraman, V. R., Stymiest, J. L., Derksen, D. J., Martin, N. I. & Vederas, J. C. Multiple on-resin olefin metathesis to form ring-expanded analogues of the lantibiotic peptide, lacticin 3147 A2. Org. Lett. 9, 699–702 (2007).
McClerren, A. L. et al. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl Acad. Sci. USA 103, 17243–17248 (2006).
Yoganathan, S. & Vederas, J. C. Fracturing rings to understand lantibiotics. Chem. Biol. 15, 999–1001 (2008).
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Research Chair in Bioorganic and Medicinal Chemistry for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the late Dr C Richard Hutchinson for his exceptional contributions to natural product biosynthesis, engineering, and drug discovery.
Rights and permissions
About this article
Cite this article
Ross, A., Vederas, J. Fundamental functionality: recent developments in understanding the structure–activity relationships of lantibiotic peptides. J Antibiot 64, 27–34 (2011). https://doi.org/10.1038/ja.2010.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2010.136
Keywords
This article is cited by
-
First evidence of production of the lantibiotic nisin P
Scientific Reports (2020)
-
High-resolution NMR studies of antibiotics in cellular membranes
Nature Communications (2018)
-
Nano-engineering the Antimicrobial Spectrum of Lantibiotics: Activity of Nisin against Gram Negative Bacteria
Scientific Reports (2017)
-
Cysteine-rich low molecular weight antimicrobial peptides from Brevibacillus and related genera for biotechnological applications
World Journal of Microbiology and Biotechnology (2017)
-
Lanthipeptides: chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production
Microbial Cell Factories (2016)