Abstract
Ribosomal 16S rRNA gene pyrosequencing was used to explore whether the genetically modified (GM) Bt-maize hybrid MON 89034 × MON 88017, expressing three insecticidal recombinant Cry proteins of Bacillus thuringiensis, would alter the rhizosphere bacterial community. Fine roots of field cultivated Bt-maize and three conventional maize varieties were analyzed together with coarse roots of the Bt-maize. A total of 547 000 sequences were obtained. Library coverage was 100% at the phylum and 99.8% at the genus rank. Although cluster analyses based on relative abundances indicated no differences at higher taxonomic ranks, genera abundances pointed to variety specific differences. Genera-based clustering depended solely on the 49 most dominant genera while the remaining 461 rare genera followed a different selection. A total of 91 genera responded significantly to the different root environments. As a benefit of pyrosequencing, 79 responsive genera were identified that might have been overlooked with conventional cloning sequencing approaches owing to their rareness. There was no indication of bacterial alterations in the rhizosphere of the Bt-maize beyond differences found between conventional varieties. B. thuringiensis-like phylotypes were present at low abundance (0.1% of Bacteria) suggesting possible occurrence of natural Cry proteins in the rhizospheres. Although some genera indicated potential phytopathogenic bacteria in the rhizosphere, their abundances were not significantly different between conventional varieties and Bt-maize. With an unprecedented sensitivity this study indicates that the rhizosphere bacterial community of a GM maize did not respond abnormally to the presence of three insecticidal proteins in the root tissue.
Similar content being viewed by others
Introduction
Genetically modified (GM) Bt-maize expresses insecticidal proteins derived from crystal delta endotoxins (Cry proteins) of Bacillus thuringiensis to become resistant against important agricultural pests. In comparison with many chemical insecticides used in agriculture, the different Cry proteins produced by B. thuringiensis (Crickmore et al., 2012) have a narrow host range. To provide broader protection, Bt-maize varieties with multiple Cry proteins have been developed. MON 89034 × MON 88017 (3BT) is a maize hybrid that encodes genes for Cry1A.105 and Cry2Ab2 for protection against the European corn borer Ostrinia nubilalis along with Cry3Bb1, which protects against the Western rootworm (Diabrotica virgifera) (EFSA Panel on Genetically Modified Organisms (GMO), 2010).
One of the major potential environmental risks associated with the use of Bt-maize varieties is their effect on soil and its inhabiting non-target organisms, including bacteria. The bacterial community inhabiting the rhizosphere, that is, the soil influenced by root metabolites, is of special importance. The easily available carbon sources exert a great selective power on the enrichment of soil bacteria and may attract both beneficial and detrimental bacteria. Differences in rhizodeposition by the plants are reflected by differently composed bacterial communities in the rhizosphere (Brimecombe et al., 2001) as they become evident between different plant species (Dohrmann and Tebbe, 2005) or cultivars grown in the same soil (Buee et al., 2009) and even across different root sections of the same plant (Watt et al., 2006).
GM Bt-maize events produce Cry proteins, typically also in their root tissue. Such recombinant products may thus potentially enter the rhizosphere as an additional nutrient source for the soil microbial community. However, studies so far indicated that alterations of the bacterial community structure of Bt-maize producing single Cry proteins were in the range of differences between conventional varieties or not detectable (Devare et al., 2004; Baumgarte and Tebbe, 2005; Miethling-Graff et al., 2010). Considering that these studies were based on classical cloning and sequencing approaches and/or on genetic fingerprinting, the lack of detection may in fact be linked to the relatively low sensitivity of such methods. Fingerprinting methods, for example, differentiate normally <100 community members (Dunbar et al., 2001; Janssen, 2006; Schütte et al., 2008; Aiken, 2011). Considering that 1 g of soil may harbor 103–106 bacterial species (Torsvik et al., 1990; Curtis et al., 2002; Gans et al., 2005; Roesch et al., 2007), the vast majority would remain undetected. Community richness estimations in fact indicated that most of the diversity in the environment is due to rare members (Hughes et al., 2001; Reeder and Knight, 2009) and these may also have important ecological functions, for example, in cycling of elements, as a supplier of phytohormones or as phytopathogens (Karadeniz et al., 2006; Humbert et al., 2010; Pester et al., 2010; Roper, 2011; Krzmarzick et al., 2012).
The aim of this study was to characterize the rhizosphere bacterial community composition of the maize hybrid MON 89034 × MON 88017 with the high resolution of 16S rRNA gene pyrosequencing. To appropriately scale potential differences caused by the genetic modification, controls included three other maize varieties cultivated on the same field and the analyses of fine and coarse roots of the Bt-maize. A special emphasis of the data analyses was made to search for unintended effects on potential plant pathogenic bacteria and the natural producer of Cry proteins, B. thuringiensis.
Materials and methods
Plants and field design
MON 89034 × MON 88017 (3BT) is a maize hybrid that produces three different insecticidal delta endotoxins Cry1A.105, Cry2Ab2 and Cry3Bb1 and the CP4 EPSPS protein conferring tolerance to the herbicidal compound glyphosate. The variety of the hybrid is DKC 5143 (NI) that was included in this study as a near isogenic counterpart in addition to two conventional varieties (Benicia (BEN), DKC 4250 (4250)). Seeds of 3BT, NI and 4250 were obtained from Monsanto Agrar GmbH (Düsseldorf, Germany), those of BEN from Pioneer Hi-Breed GmbH (Buxtehude, Germany). The maize plants were cultivated on a field site at the vTI research center in Braunschweig, Germany; soil properties (site BS-1) have been described elsewhere (Castellanos et al., 2009). The field consisted of a randomized block design including 40 plots with maize and 8 replicates for each variety with NI treated or not treated with a conventional insecticide (not analyzed in this study). The field was arranged in five rows of eight plots, each of 1260 m2 area. The whole experimental field was bordered by an 8-m wide strip with maize 4250. All maize varieties were sown on 18 May 2009.
Sample collection and preparation of molecular analyses
At the flowering stage, four plants of each variety were carefully dug out of each plot and transferred immediately to the laboratory. Loosely adhering soil was removed by shaking the roots and subsequently dipping them into sterile saline (0.85% NaCl (wt/vol)). The roots of each individual plant were treated as independent replicates and they were divided into fractions of fine roots that were <1 mm in diameter and coarse roots with 2–4-mm diameter. Bacterial cells adhering to the roots were detached by suspending 3 g of fresh root material in 30 ml of sterile saline for 30 min at 4 °C in an orbital shaker (Model 3040, GFL, Burgwedel, Germany) at 10 r.p.m. The washing solution was divided into two aliquots and the microbial cells were collected by centrifugation at 4100 × g for 30 min at 4 °C. The pellets were stored at −70 °C. In parallel, samples of the washed root fractions were stored at −70 °C to later quantify Cry proteins by enzyme-linked immunosorbent assay as described in the Supplemental Material. It was confirmed that the Cry proteins were only produced by 3BT but not by the conventional varieties. The contents of single Cry proteins ranged from 3 to 12‰ (w/w total protein).
DNA extraction and purification
DNA was extracted from the frozen cell pellets using the ‘FastDNA SPIN kit for soil’ (MP Biomedicals, Illkirch, France). The extraction included two bead beating steps of 45 s at 6.5 m s−1 on a FastPrep-24 system (MP Biomedicals) and three additional washing steps of the binding matrix each with 1 ml 5.5 M guanidine thiocyanate (Carl Roth, Karlsruhe, Germany) until the matrix retained its original color. An aliquot of cells yielded 100 μl of DNA-solution with ∼20 ng DNA per μl from fine and 40 ng DNA per μl from coarse roots.
Quantification of the bacterial community
Population sizes of the rhizosphere bacterial communities were determined by quantitative real-time PCR applying the ABsolut QPCR SYBR Green Fluorescein mix (Thermo Fisher Scientific, Epsom, UK) and 0.3 μM of each of the universal bacterial primers F27 (5′-AGAGTTTGATCMTGGCTCAG-3′ (Lane, 1991)) and Eub338rev (5′-CTGCTGCCTCCCGTAGGAGT-3′ (Lane, 1991)) that successfully excluded genomic 18S rRNA genes of potentially contaminating maize root cells. A total of 2 μl of template DNA diluted 10-fold in TE-buffer (10 mM Tris, 1 mM EDTA, pH 8) were used in 25 μl reaction volume. All communities were analyzed in duplicates and amplification was carried out in a Bio-Rad MyiQ iCycler (Bio-Rad Laboratories GmbH, München, Germany). PCR started with 15 min at 95 °C, then 40 cycles of 35 s at 94 °C, 35 s at 57 °C, 45 s at 72 °C, 15 s at 83 °C and finally 5 min at 72 °C. Standard curves were obtained from 10-fold dilutions of the pGEM-T vector (Promega, Mannheim, Germany) containing the 16S rRNA gene of Bacillus subtilis BD466 (Escherichia coli positions 8–1513 (Brosius et al., 1981)). The average PCR efficiency was 97% with an R2 of the standard curves of 0.99.
Pyrosequencing and sequence processing
Bacterial communities obtained from fine roots of the four maize varieties BEN, 4250, NI and 3BT, as well as from coarse roots of 3BT, were selected for pyrosequencing. A pre-screening by terminal-restriction fragment length polymorphism of the bacterial communities from five replicate field plots of each variety helped to select the two most dissimilar field plots, to account for variation due to field heterogeneity. The two plots were located ∼230 m away from each other. Thus, in total, 10 independent samples were analyzed and compared with each other (Table 1). These 10 samples were tagged by different multiplex identifiers integrated into the sequence of the forward primers. The multiplex identifiers were selected from the multiplex identifier standard 454 set (Roche, Mannheim, Germany) that is a set of 10-mer sequences carefully engineered to avoid misassignment of reads and that are tolerant to several errors like insertions, deletions or substitutions. DNA extracts of the four replicate plants of each plot (biological replicates) were amplified separately but could not be distinguished later on. A 408-bp segment of the 16S rRNA genes spanning E. coli positions 519–926 was suitable for pyrosequencing (Youssef et al., 2009) and PCR amplified with universal bacterial primers Com1 and Com2 (Schwieger and Tebbe, 1998). These primers were modified to perform pyrosequencing on the GS FLX Titanium system (Roche) applying the Lib-L emulsion PCR method. Full primer sequences are given in Supplementary Table S1. Each DNA extract was amplified separately applying one forward primer (0.5 μM), the reverse primer (0.5 μM), 0.2 mM of each dNTP, 2% dimethyl sulfoxide and 2.5 U 100 μl−1 FastStart High Fidelity enzyme blend (Roche) in a 1x reaction buffer including 1.8 mM MgCl2. A total of 1 μl template DNA was added to a final volume of 30 μl reaction mix. PCR conditions were 15 min at 95 °C, 30 cycles of 94 °C for 60 s, 50 °C for 60 s, 72 °C for 70 s; and 5 min at 72 °C. Products from three independent replicate amplifications (technical replicates) were pooled and purified from agarose gels following the respective protocol of the PCR Clean-Up and Gel-Extraction System (SLG, Gauting, Germany) and quantified with the Quant-iT PicoGreen dsDNA assay (Invitrogen, Darmstadt, Germany). Equimolar amounts of the 40 individual PCR products were pooled for pyrosequencing. Sequence data were processed by the RDP’s pyrosequencing pipeline (Ribosomal Database Project, pyro.cme.msu.edu (Cole et al., 2009)) as described in Supplemental Material.
Bioinformatic analyses for higher taxonomic ranks (phylum to genus) and at the level of operational taxonomic units
Detailed information on the formation of operational taxonomic units (OTUs) that combine sequences of >97% similarity, on the calculation of the library coverage C (Good, 1953), the Shannon diversity index H’ (Shannon and Weaver, 1963) and the species evenness J’ (Pielou, 1966) are given in the Supplemental Material. This also includes information on comparisons applying the Student’s t-test, analysis of variance or BioNumerics 5.10 for cluster analyses (Applied Maths, Sint-Martens-Latem, Belgium), as well as information on the search for B. thuringiensis and plant pathogenic bacteria.
Deposition of DNA sequences
From pyrosequencing of bacterial 16S rRNA genes 604 400 sequences were obtained of which 546 941 were retained as high-quality sequences. All sequences retrieved and analyzed in this study have been deposited to the Sequence Read Archive under the study accession number ERP001118 (http://www.ebi.ac.uk/ena/data/view/ERP001118).
Results
Bacterial population size in the maize rhizospheres
Quantitative PCR of the 16S rRNA genes retrieved between 2 and 5 × 105 copy numbers per ng DNA from the rhizosphere samples (Supplementary Figure S1), indicating that bacterial abundance was not affected by the variety including 3BT, which produced Cry proteins in their root tissue. Differences in relative abundance of DNA sequences found between the varieties in the subsequent pyrosequencing were therefore directly comparable.
DNA sequence distribution
A total of 546 941 DNA sequences were obtained by pyrosequencing of bacterial 16S rRNA genes. Amplicons were evenly distributed among the 10 communities analyzed, ranging from 8.1 to 12.7% for the single samples, except for 3BT_c that was underrepresented with 4.9% of the total sequences (Supplementary Table S2a). Approximately 0.5% of the total amplicons originated from Archaea and most of them were affiliated to the class Thermoprotei (Crenarchaeota; data not shown). The proportion of the bacterial sequences that could be assigned to the different taxonomic ranks declined with increasing discriminatory taxonomic resolution: Although 88% of the bacterial sequences could be assigned to phyla, 86% fell into classes, 71% into orders, 59% into families and 46% into genera, respectively.
On average, the sequences of a single sample were composed of 19±1 different phyla, 41±2 classes, 45±4 orders, 120±7 families and 324±25 genera (Supplementary Table S2b). Considering all 10 maize rhizosphere bacterial communities together, 22 phyla, 48 classes, 60 orders, 159 families and 510 genera were detected, indicating a great overlap of the detected taxonomic units in all samples at the higher hierarchical ranks, but a lower overlap at the genus rank. The library coverage C of the taxonomic ranks from phylum to family was above 99.9% and slightly lower (99.7%) at the rank genus (Supplementary Table S3), suggesting that in all 10 samples, including the underrepresented sample 3BT_c, the vast majority of all taxonomic units was detected. Yet, rarefaction analyses based on OTUs, that joined sequences of >97% similarity, indicated that the communities were still not sampled to saturation at this respective taxonomic rank (Supplementary Figure S2). The rarefaction curves of all samples followed similar progressions, indicating that the communities were of comparable diversity. This was also stressed by their similar Shannon indices H’ with an average of 7.66±0.40 and the average species evenness J’ of 0.83±0.01 (Supplementary Table S4).
Taxonomic assignment of the 16S rRNA gene sequences
For all hierarchical ranks of the taxonomic system, considering phylum to genus, the same units were detected from the 10 different samples and they ranked in their abundances at similar positions. Among the Bacteria, the most abundant phyla were Proteobacteria and Actinobacteria with almost 40% and 30% of the sequences, respectively (Table 2a). The most represented classes were Actinobacteria, Betaproteobacteria and Alphaproteobacteria, which together comprised >60% of all bacterial amplicons (Table 2b). Among the 510 genera, the most dominant 108 genera were present in all rhizosphere samples, these included Streptomycetes, Nocardioides, Massilia and Gp6 (Acidobacteria; Table 2c). Only nine genera were represented by high sequence numbers (>1% of Bacteria) while most of the genera (300) were represented by very low sequence loads (<0.01%; Figure 1a). This pattern of mapping few genera to the group with high sequence load and most of the genera to the group with low load was not only seen on the total bacterial diversity but also within the 5 phyla represented by 20 or more genera. Among the 300 genera with very low sequence numbers, 112 genera were present in at least 5 of 10 samples, while 81 were only present in a single sample and 55 genera were only represented by 1 single sequence. The total bacterial abundance of these 300 genera summed up to only 1.8%, whereas the contribution of the 9 genera represented by a higher number of sequences (>1%) was 17% (Figure 1b).
Relative sequence load of genera. Sequence load of 100% correspond to all Bacteria-assigned sequences of a sample. Percent-values specify averages of all 10 samples. The general distribution of sequence loads of all genera is displayed next to those from most abundant five phyla. Number of genera in respect to their relative sequence load (a). Sum of relative abundances of all genera assigned to the respective groups of sequence load (b).
Identification of shared OTUs
For the identification of OTUs that combine sequences from all 10 samples with sequence similarities of >97%, all the sequences of each sample were separately analyzed to form basic OTUs and one representative sequence was selected for each basic OTU. These 106 090 representatives were then combined for the calculation of a second analysis generating 61 067 superior OTUs. It turned out that the dominant superior OTUs were in fact shared among all samples (Table 3). Most sequences from the dominant 40 superior OTUs were assigned to Methylibium sp. TPD48 (OTU 14) and to Streptomyces achromogenes (OTU 2), respectively. In both cases the sequences were distributed among three or four superior OTUs, but because their representatives shared >97% sequence identity, they could not be assigned to different ‘species’. Such sequence variability indicated a great diversity at the taxonomic scale below the species level (>97% sequence similarity).
Similarities between independent replicates illustrating spatial variability
The variability of rhizosphere bacterial communities from the two replicate field plots was the basis of this study for each root environment. Despite the spatial distance of 230 m, high similarities of relative abundance pattern were obtained for the five pairs of replicate communities, that is, the fine roots of the four maize varieties and the coarse roots of 3BT, at all hierarchical taxonomic ranks (Supplementary Figure S3). The similarities between replicates decreased with increasing taxonomic resolution. Pearson’s correlation coefficients revealed average similarities of 99.0±0.7% on relative phyla abundances between each pair of replicate samples, but at the rank genus similarities were 93.6±1.4%. The less represented sample 3BT_c correlated well with its replicate sample 3BT_d even though it contained 57% less sequences and thus all communities were well suitable for comparison.
Effect of the variety and the root microhabitat (fine versus coarse roots)
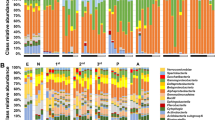
Cluster analyses on relative abundance patterns showed for all hierarchical ranks, from phyla down to genera, that the rhizosphere bacterial communities of all four varieties were highly similar. Similarities were at least 92% for the genera and 100% for the phyla (Supplementary Figure S4). Clustering of replicates into groups according to the maize variety was not seen on the basis of phyla (Supplementary Figure S4a), classes (Supplementary Figure S4b) or orders (Supplementary Figure S4c). At the family (Supplementary Figure S4d) and genus ranks (Figure 2a), however, distinct bacterial compositions were revealed for the conventional variety BEN but not for the GM variety 3BT, or any other. Separation of bacterial communities from fine or coarse roots of 3BT only became visible at the rank genus, while at the higher taxonomic ranks the effect of the two replicate field plots was stronger than the respective root microhabitat. In fact, fine and coarse roots of the same field plot clustered together at the ranks of phyla, classes and orders, respectively. A stepwise reduction of the genera abundance pattern by the less represented genera revealed that solely the dominant 49 most represented genera, which each contributed >0.5% of the genera-assigned sequences (that is, >0.23% of all Bacteria-assigned sequences as only 46% of Bacteria were assigned to genera; Figure 2b and Supplementary Table S5), were necessary to separate the samples in the same manner as all 510 genera did (Figure 2a). These dominant 49 genera (in the following referred as the ‘dominant genera’) represented 10% of the genera richness but 76% of the genera-assigned sequences. The remaining less represented 461 genera (referred to as the ‘rare genera’) did not affect the clustering; on the contrary, they clustered in a different manner with no evidence that the plant variety or the root microhabitat was influential (Figure 2c).
Cluster analyses (UPGMA) of the rhizosphere bacterial communities from different maize varieties and microhabitats (fine roots, coarse roots) based on the relative abundances of all genera (a), the 49 most dominant genera that each represent >0.5% of all genera-assigned sequences (each genus with >0.23% of all Bacteria) (b), the remainig lower represented 461 genera (c) and the 91 genera significantly responsive to the root environments. Relative abundances were normalized for each comparison, respectively. Pearson’s correlation on relative taxa abundances comprising all detected taxa of a given rank.
To evaluate whether distinct taxa were preferred among the dominant genera, the taxonomic assignment to classes of the 49 dominant genera was compared with that of all 510 genera (Supplementary Table S6). Although for most classes the proportion of contributing genera was similar in both groups, the percentage of dominant genera belonging to the Betaproteobacteria was notably higher (18%) than that of all detected genera (11%). In contrast, Deltaproteobacteria and Clostridia were not represented by the dominant genera even though they comprised 4% and 10% of all detected genera, respectively.
Similarity values of bacterial communities based on all 510 genera were calculated for all possible comparisons of any two varieties: At the higher hierarchical taxonomic ranks, the similarity values were not distinguishable from each other, but when genera or family abundances were taken into consideration (Figure 3a), the varieties BEN and NI selected for the most distinct bacterial communities. In contrast, NI and its GM version 3BT were the most similar varieties at these ranks. Thus, the effect of 3BT was not greater than that of the other varieties. At all taxonomic ranks the effect of the varieties was in the range of the effect of the root microhabitat (created by fine or coarse roots), as indicated by the fact that the similarity values were not significantly different. To specifically assess the GM variety 3BT in the background of the three conventional varieties, relative abundance patterns were compared with each other either excluding the GM variety 3BT or including it. A decline in similarity values lower than those found between the three conventional varieties would indicate greater differences caused by the GM variety 3BT but such reduced similarity values were not obtained at any of the taxonomic ranks analyzed (Figure 3b).
Similarities of the rhizosphere bacterial communities at different taxonomic ranks between (a) two varieties as indicated or (b) the three conventional varieties (three varieties) or the three conventional varieties and 3BT (four varieties). Significance of difference was tested by analysis of variance, P⩽0.05. Comparisons were taken at each taxonomic rank separately and only differences were labeled in the figure. Bars with no significant difference to each other are indicated by the same letter.
Comparing single genera abundances identified 91 genera that significantly responded in its abundance to one variety in comparison with its abundance in all other varieties or to the root microhabitat (Table 4). Most genera were affected by BEN (31 genera) and 4250 (26), whereas the GM variety 3BT and its near isogenic counterpart NI affected only 14 and 17 genera, respectively. A total of 14 genera were also differently abundant on fine and coarse roots of 3BT. It should be noted that the identification of responsive genera might potentially be biased by low abundant genera due to their stochastic presence in some but not in all samples. However, in these data, such a bias was not indicated because the fraction of genera with a sequence load<0.01% of Bacteria was 59% of all detected genera and only 43% of the responsive genera (Supplementary Figure S5). In addition, specific effects of the varieties mostly caused increases of omnipresent genera rather than decreases. Only 12 responsive genera referred to the 49 dominant genera identified above (Supplementary Table S5). These dominant genera were sensitive to BEN, 4250, NI or the microhabitat of the root, while only less represented genera responded to 3BT. The strongest response to a maize variety was recorded in the rhizosphere of BEN where the genus Pantoea was 42-fold more abundant compared with the other varieties. However, because of the low overall abundance of this genus, the change in absolute sequence numbers was still small. Greatest changes, of >100 sequences difference (corresponding to an average difference of >0.18%), were only found for 10% of the genera and restricted to the already high abundant genera like Massilia that was 0.4-fold reduced or Burkholderia that increased fivefold in the rhizosphere of BEN. The responsive genera in the rhizosphere of 3BT indicated no explicit growth on the additional Cry proteins. On the other hand, two genera involved in nitrification were significantly more abundant: Although Nitrosomonas was present at the minimum amount of detection (0.007%), Nitrospira was more abundant (0.067%) and present in all rhizosphere samples.
A cluster analysis based on solely the relative abundances of the 91 responsive genera, yielded a much clearer cluster formation than analyses including all genera (Figure 2d): Specific bacterial communities for BEN and 4250 were indicated by separate clusters, but NI as well as fine and coarse roots of 3BT clustered closely together. These demonstrated rather small differences between the GM variety 3BT and its near isogenic counterpart while the effects of 4250 or BEN in correlation to the other two varieties were greater. The taxonomic affiliation of the 91 responsive genera (Supplementary Table S6) correlated well with the distribution of all 510 genera to their classes. Clostridia, a class with endospore forming bacteria, was apparently more resistant to changes in the rhizosphere (5% of responsive and 10% of all detected genera), suggesting that also metabolically inactive spores were detected.
Search for B. thuringiensis and potential bacterial plant pathogens
The potential presence of a B. thuringiensis-like phylotype was indicated for an average of 0.12% of all Bacteria assigned 16S rRNA genes that were highly (⩾97% sequence identity) similar to strains of B. thuringiensis (Table 5). Their abundance was not statistically different between the varieties. Concerning potential bacterial plant pathogens, not a single sequence affiliated to the genera Erwinia, Xanthomonas or Xylella (limit of detection: one sequence in a minimum background of 26 670 and a maximum of 68 751 sequences). Two single sequences highly similar to Xylophilus ampelinusi were detected in data sets of 4250_a and 3BT_c. Also, highly similar sequences to Pantoea stewartii were present at the threshold of detection in 5 of the 10 samples analyzed. Higher abundances in maize rhizospheres were found for Pseudomonas syringae and Ralstonia solanacearum phylotypes with average rates of 0.13% and 0.03%, respectively. Abundances of these bacteria were not significantly affected by the genetic modification of 3BT. However, it should be stressed that pathogenicity cannot be solely identified based on 16S rRNA gene sequence similarities.
Discussion
More than 500 000 bacterial 16S rRNA genes were retrieved from the rhizosphere of field-grown maize in this study and the taxonomic assignment of these sequences revealed the same dominant bacterial groups that have already been detected in similar studies based on much smaller sequence numbers (Chelius and Triplett, 2001; Schmalenberger and Tebbe, 2002; Castellanos et al., 2009). This consistency suggests that generally maize selects independent of its variety, age or soil in which it was cultivated for a similar core bacterial community, which is also quite similar to dominant members from other plant species (Buee et al., 2009; da Rocha et al., 2009). In contrast to the low-throughput sequencing methods that are typically limited to the most dominant 100 phylotypes (rRNA genes; Dunbar et al., 2001; Janssen, 2006; Schütte et al., 2008; Aiken, 2011), the pyrosequencing applied here detected 510 different genera and 61 000 superior OTUs, thus, opening the view on the less abundant, ‘rare’ community members by a factor of more than two orders of magnitude. Library coverage values of >99% for the hierarchical ranks from phyla down to genera indicated that the largest part of the actual bacterial diversity was captured in this study with sample sizes in the range of 50 000 sequences from a single rhizosphere.
The different maize varieties analyzed in this study harbored the same bacterial population sizes in their rhizosphere and cluster analyses revealed that the bacterial diversity of the maize varieties, including 3BT, was generally not distinguishable at the taxonomic ranks down to families. With the exception of BEN this was also true at the rank genus. However, at the rank genus differences were detected between bacterial communities from fine and coarse roots, which confirms inhomogeneous colonization of root segments (Watt et al., 2006; DeAngelis et al., 2009). Only the community structure of the 49 most dominant genera was necessary to obtain exactly the same clustering as with all 510 genera. In contrast, the community represented only by the 461 less abundant genera followed a different selection. They either responded to substrates that were not variety specific or they were not much influenced by the rhizosphere and more dependent on other factors, for example, heterogeneities in soil properties (Berg and Smalla, 2009). Only 12 of the 91 responsive genera identified in this study belonged to the dominant genera, whereas 79 were assigned to the rare. Thus, even though the community of the rare genera in general did not respond to the different root environments, individual members did. Accordingly, the inconsistencies in the detection of effects of Bt-maize events on the rhizosphere bacterial communities by low-throughput approaches (Baumgarte and Tebbe, 2005; Miethling-Graff et al., 2010) might be caused by sensitive members with abundances in the border zone of the detection threshold of the respective methods. In this study, the identification of 87% of the responsive genera was clearly the benefit of the high-throughput approach, making it an important tool for future community analyses. Once, genera are identified as responsive with regard to the different conditions in the rhizospheres, they require specific attention as they might bear the potential to act as indicators.
The roots of the GM 3BT contain Cry proteins that may get into contact with rhizosphere bacteria. Although it is still debatable if Cry proteins are in fact exudated by the roots (Saxena et al., 2004; Miethling-Graff et al., 2010), its release during root growth by the sloughing off of living cells or from wounded cells is very likely (Faure et al., 2009) and thus exposure to bacteria on the root surface can be expected. Considering the small proportion of Cry proteins in the total protein contents of the root cells (maximum ∼3% (w/w); see Materials and methods) and the lack of direct toxic effects on soil bacteria in general (Icoz and Stotzky, 2008), shifts in the bacterial community structure triggered by 3BT are expected to be minor. Interestingly, however, Nitrosomonas and Nitrospira, two genera involved in nitrification, were in fact slightly increased in the rhizosphere of 3BT. Assuming that protein degradation will lead to amino acids from which finally ammonia could be released, the increase in nitrifying bacteria might indicate degradation of Cry proteins. Further studies would be needed to evaluate whether this observation is in fact linked to the genetic modification or not.
In summary, the application of 16S rRNA gene pyrosequencing confirmed patterns of dominant bacteria from previous low-throughput bacterial community analyses in maize rhizospheres and indicated only marginal effects of the different root environments, as provided by the different varieties, including the GM 3BT, or the root segment. The high-throughput approach applied here allowed identifying 91 bacterial genera as responsive to the specific root environments of which 87% would not have been identified by traditional cloning and sequencing community analyses given their respective detection limits. Bacteria from these responsive genera appear to interact highly specific with particular cultivars and/or root segments and thus deserve future investigation.
Accession codes
References
Aiken JT . (2011). Terminal restriction fragment length polymorphism for soil microbial community fingerprinting. Soil Sci Soc Am J 75: 102–111.
Baumgarte S, Tebbe CC . (2005). Field studies on the environmental fate of the Cry1Ab Bt-toxin produced by transgenic maize (MON810) and its effect on bacterial communities in the maize rhizosphere. Mol Ecol 14: 2539–2551.
Berg G, Smalla K . (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68: 1–13.
Brimecombe MJ, De Leij FA, Lynch JM . (2001). The effect of root exudates on rhizosphere microbial population. In:Pinton R, Varanini Z, Nannipieri P, (eds) The Rhizosphere. Marcel Dekker, Inc: New York, NY, pp 95–140.
Brosius J, Dull TJ, Sleeter DD, Noller HF . (1981). Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol 148: 107–127.
Buee M, de Boer W, Martin F, van Overbeek L, Jurkevitch E . (2009). The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 321: 189–212.
Castellanos T, Dohrmann AB, Imfeld G, Baumgarte S, Tebbe CC . (2009). Search of environmental descriptors to explain the variability of the bacterial diversity from maize rhizospheres across a regional scale. Eur J Soil Biol 45: 383–393.
Chelius MK, Triplett EW . (2001). The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb Ecol 41: 252–263.
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ et al (2009). The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–D145.
Crickmore N, Zeigler DR, Schnepf E, Van Rie J, Lereclus D, Baum J et al (2012) Bacillus Thuringiensis Toxin Nomenclaturehttp://www.lifesci.sussex.ac.uk/Home/Neil_Crickmore/Bt/.
Curtis TP, Sloan WT, Scannell JW . (2002). Estimating prokaryotic diversity and its limits. Proc Natl Acad Sci USA 99: 10494–10499.
da Rocha UN, van Overbeek L, van Elsas JD . (2009). Exploration of hitherto-uncultured bacteria from the rhizosphere. FEMS Microbiol Ecol 69: 313–328.
DeAngelis KM, Brodie EL, DeSantis TZ, Andersen GL, Lindow SE, Firestone MK . (2009). Selective progressive response of soil microbial community to wild oat roots. ISME J 3: 168–178.
Devare MH, Jones CM, Thies JE . (2004). Effect of Cry3Bb transgenic corn and tefluthrin on the soil microbial community: biomass, activity, and diversity. J Environ Qual 33: 837–843.
Dohrmann AB, Tebbe CC . (2005). Effect of elevated tropospheric ozone on the structure of bacterial communities inhabiting the rhizosphere of herbaceous plants native to Germany. Appl Environ Microbiol 71: 7750–7758.
Dunbar J, Ticknor LO, Kuske CR . (2001). Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl Environ Microbiol 67: 190–197.
EFSA Panel on Genetically Modified Organisms (GMO) (2010). Scientific opinion on application (EFSA-GMO-NL-2007-39) for the placing on the market of insect resistant and herbicide tolerant genetically modified maize MON89034 x MON88017 for food and feed uses, import and processing under regulation (EC) no 1829/2003 from Monsanto. EFSA J 8: 1564.
Faure D, Vereecke D, Leveau JH . (2009). Molecular communication in the rhizosphere. Plant Soil 321: 279–303.
Gans J, Wolinsky M, Dunbar J . (2005). Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309: 1387–1390.
Good IJ . (1953). The population frequencies of species and the estimation of population parameters. Biometrika 40: 237–264.
Hughes JB, Hellmann JJ, Ricketts TH, Bohannan BJM . (2001). Counting the uncountable: Statistical approaches to estimating microbial diversity. Appl Environ Microbiol 67: 4399–4406.
Humbert S, Tarnawski S, Fromin N, Mallet MP, Aragno M, Zopfi J . (2010). Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J 4: 450–454.
Icoz I, Stotzky G . (2008). Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biol Biochem 40: 559–586.
Janssen PH . (2006). Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 72: 1719–1728.
Karadeniz A, Topcuoglu S, Inan S . (2006). Auxin, gibberellin, cytokinin and abscisic acid production in some bacteria. World J Microbiol Biotechnol 22: 1061–1064.
Krzmarzick MJ, Crary BB, Harding JJ, Oyerinde OO, Leri AC, Myneni SC et al (2012). Natural niche for organohalide-respiring Chloroflexi. Appl Environ Microbiol 78: 393–401.
Lane DJ . (1991). 16S/23S rRNA sequencing. In:Stackebrandt E, Goodfellow M, (eds) Nucleic Acid Techniques in Bacterial Systematics. John Wiley and Sons: Chichester, UK, pp 115–175.
Miethling-Graff R, Dockhorn S, Tebbe CC . (2010). Release of the recombinant Cry3Bb1 protein of Bt maize MON88017 into field soil and detection of effects on the diversity of rhizosphere bacteria. Eur J Soil Biol 46: 41–48.
Pester M, Bittner N, Deevong P, Wagner M, Loy A . (2010). A ‘rare biosphere’ microorganism contributes to sulfate reduction in a peatland. ISME J 4: 1591–1602.
Pielou EC . (1966). Measurement of diversity in different types of biological collections. J Theor Biol 13: 131–144.
Reeder J, Knight R . (2009). The ‘rare biosphere’: a reality check. Nature Meth 6: 636–637.
Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AK, Kent AD et al (2007). Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1: 283–290.
Roper MC . (2011). Pantoea stewartii subsp. stewartii: lessons learned from a xylem-dwelling pathogen of sweet corn. Mol Plant Pathol 12: 628–637.
Saxena D, Stewart CN, Altosaar I, Shu QY, Stotzky G . (2004). Larvicidal Cry proteins from Bacillus thuringiensis are released in root exudates of transgenic B. thuringiensis corn, potato, and rice but not of B. thuringiensis canola, cotton, and tobacco. Plant Physiol Biochem 42: 383–387.
Schmalenberger A, Tebbe CC . (2002). Bacterial community composition in the rhizosphere of a transgenic, herbicide-resistant maize (Zea mays) and comparison to its non-transgenic cultivar Bosphore. FEMS Microbiol Ecol 40: 29–37.
Schütte UM, Abdo Z, Bent SJ, Shyu C, Williams CJ, Pierson JD et al (2008). Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Appl Microbiol Biot 80: 365–380.
Schwieger F, Tebbe CC . (1998). A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl Environ Microbiol 64: 4870–4876.
Shannon CE, Weaver W . (1963) The mathematical theory of communication. University of Illinois Press: Urbana, Illinois.
Torsvik V, Goksoyr J, Daae FL . (1990). High diversity in DNA of soil bacteria. Appl Environ Microbiol 56: 782–787.
Watt M, Hugenholtz P, White R, Vinall K . (2006). Numbers and locations of native bacteria on field-grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environ Microbiol 8: 871–884.
Youssef N, Sheik CS, Krumholz LR, Najar FZ, Roe BA, Elshahed MF . (2009). Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16S rRNA gene-based environmental surveys. Appl Environ Microbiol 75: 5227–5236.
Acknowledgements
We thank Florian Hackelsperger and his colleagues at the Experimental Research Station (FLI, Braunschweig) for seeding and managing the experimental field site. We gratefully acknowledge Martin Bolz (vTI, IT Facility Services) for maintenance of computer systems and Claudia Wiese, Jana Usarek, Karin Trescher (vTI, Institute for Biodiversity) for the excellent technical assistance and all members of the joint research project ‘Biosafety field studies on maize with multiple genes providing protection against the European corn borer and the Western rootworm’, especially Ingolf Schuphan and Stefan Rauschen for collaboration. The work was financially supported by the BMBF project numbers 0315215C. Sebastian Jaenicke acknowledges funding by the German Federal Ministry of Education and Research (GenoMik-Transfer, Grants 0315599A and 0315599B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Dohrmann, A., Küting, M., Jünemann, S. et al. Importance of rare taxa for bacterial diversity in the rhizosphere of Bt- and conventional maize varieties. ISME J 7, 37–49 (2013). https://doi.org/10.1038/ismej.2012.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2012.77
Keywords
This article is cited by
-
Combined metagenomic and metabolomic analyses reveal that Bt rice planting alters soil C-N metabolism
ISME Communications (2023)
-
Different soil salinity imparts clear alteration in rhizospheric bacterial community dynamics in rice and peanut
Archives of Microbiology (2022)
-
Local domestication of soybean leads to strong root selection and diverse filtration of root-associated bacterial communities
Plant and Soil (2022)
-
Small-Scale Variability in Bacterial Community Structure in Different Soil Types
Microbial Ecology (2021)
-
Plant-assisted selection: a promising alternative for in vivo identification of wheat (Triticum turgidum L. subsp. Durum) growth promoting bacteria
Plant and Soil (2019)