Abstract
Background:
Maternal obesity increases offspring propensity to metabolic dysfunctions and to non-alcoholic fatty liver disease (NAFLD), which may lead to cirrhosis or liver cancer. The circadian clock is a transcriptional/epigenetic molecular machinery synchronising physiological processes to coordinate energy utilisation within a 24-h light/dark period. Alterations in rhythmicity have profound effects on metabolic pathways, which we sought to investigate in offspring with programmed NAFLD.
Methods:
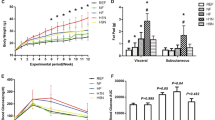
Mice were fed a standard or an obesogenic diet (OD), before and throughout pregnancy, and during lactation. Offspring were weaned onto standard or an OD at 3 weeks postpartum and housed in 12:12 light/dark conditions. Biochemical and histological indicators of NAFLD and fibrosis, analysis of canonical clock genes with methylation status and locomotor activity were investigated at 6 months.
Results:
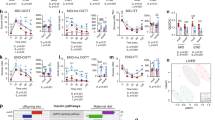
We show that maternal obesity interacts with an obesogenic post-weaning diet to promote the development of NAFLD with disruption of canonical metabolic rhythmicity gene expression in the liver. We demonstrate hypermethylation of BMAL-1 (brain and muscle Arnt like-1) and Per2 promoter regions and altered 24-h rhythmicity of hepatic pro-inflammatory and fibrogenic mediators.
Conclusions:
These data implicate disordered circadian rhythms in NAFLD and suggest that disruption of this system during critical developmental periods may be responsible for the onset of chronic liver disease in adulthood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bohinc BN, Diehl AM . Mechanisms of disease progression in NASH: new paradigms. Clin Liver Dis 2012; 16: 549–565.
Diehl AM . Neighborhood watch orchestrates liver regeneration. Nat Med 2012; 18: 497–499.
Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 2010; 52: 913–920.
Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008; 51: 383–392.
Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 2013; 58: 128–138.
Bass J, Takahashi JS . Circadian integration of metabolism and energetics. Science 2010; 330: 1349–1354.
Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 2012; 485: 123–127.
Kohsaka A, Bass J . A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab 2007; 18: 4–11.
Schibler U, Ripperger J, Brown SA . Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 2003; 18: 250–260.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308: 1043–1045.
Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007; 6: 414–421.
Liu S, Brown JD, Stanya KJ, Homan E, Leidl M, Inouye K et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 2013; 502: 550–554.
Tong X, Yin L . Circadian rhythms in liver physiology and liver diseases. Compr Physiol 2013; 3: 917–940.
Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem 2003; 278: 41519–41527.
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002; 109: 307–320.
Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006; 126: 801–810.
Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 2006; 55: 962–970.
Tevy MF, Giebultowicz J, Pincus Z, Mazzoccoli G, Vinciguerra M . Aging signaling pathways and circadian clock-dependent metabolic derangements. Trends Endocrinol Metab 2013; 24: 229–237.
Dolatshad H, Cary AJ, Davis FC . Differential expression of the circadian clock in maternal and embryonic tissues of mice. PLoS One 2010; 5: e9855.
Sumova A, Sladek M, Polidarova L, Novakova M, Houdek P . Circadian system from conception till adulthood. Prog Brain Res 2012; 199: 83–103.
Eckel-Mahan K, Sassone-Corsi P . Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol 2009; 16: 462–467.
Mazzoccoli G, Pazienza V, Vinciguerra M . Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol Int 2012; 29: 227–251.
Borengasser SJ, Kang P, Faske J, Gomez-Acevedo H, Blackburn ML, Badger TM et al. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS One 2014; 9: e84209.
Milagro FI, Gomez-Abellan P, Campion J, Martinez JA, Ordovas JM, Garaulet M . CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int 2012; 29: 1180–1194.
Folch J, Lees M, Sloane Stanley GH . A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497–509.
Rappa F, Greco A, Podrini C, Cappello F, Foti M, Bourgoin L et al. Immunopositivity for histone macroH2A1 isoforms marks steatosis-associated hepatocellular carcinoma. PLoS One 2013; 8: e54458.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321.
Orozco-Solis R, Matos RJ, Guzman-Quevedo O, Lopes de Souza S, Bihouee A, Houlgatte R et al. Nutritional programming in the rat is linked to long-lasting changes in nutrient sensing and energy homeostasis in the hypothalamus. PLoS One 2010; 5: e13537.
Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004; 2: e377.
Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 2005; 102: 12071–12076.
Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 2010; 16: 1152–1156.
Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab 2010; 12: 509–520.
Ji Y, Qin Y, Shu H, Li X . Methylation analyses on promoters of mPer1, mPer2, and mCry1 during perinatal development. Biochem Biophys Res Commun 2010; 391: 1742–1747.
Ge ZJ, Liang QX, Luo SM, Wei YC, Han ZM, Schatten H et al. Diabetic uterus environment may play a key role in alterations of DNA methylation of several imprinted genes at mid-gestation in mice. Reprod Biol Endocrinol 2013; 11: 119.
Acknowledgements
We thank Jahm Persaud, Department of Clinical Biochemistry Royal Free Hospital, UCLH/UCL Comprehensive Biomedical Research Centre, for his invaluable help. We also thank Joaquim Pombo and Paul Seed, Women’s Health Academic Centre, King’s College London, for technical assistance. This study was supported by Wellcome Trust and Fiorina Elliot Fellowship to JAO and My First AIRC Grant to MV (n.13419).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mouralidarane, A., Soeda, J., Sugden, D. et al. Maternal obesity programs offspring non-alcoholic fatty liver disease through disruption of 24-h rhythms in mice. Int J Obes 39, 1339–1348 (2015). https://doi.org/10.1038/ijo.2015.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.85
This article is cited by
-
Deficiency of histone variant macroH2A1.1 is associated with sexually dimorphic obesity in mice
Scientific Reports (2023)
-
Human centenarian–associated SIRT6 mutants modulate hepatocyte metabolism and collagen deposition in multilineage hepatic 3D spheroids
GeroScience (2023)
-
Obesity during pregnancy results in maternal intestinal inflammation, placental hypoxia, and alters fetal glucose metabolism at mid-gestation
Scientific Reports (2019)
-
Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring
Scientific Reports (2018)
-
Maternal obesity alters endoplasmic reticulum homeostasis in offspring pancreas
Journal of Physiology and Biochemistry (2016)