Abstract
Background:
Activation of the ileal brake, by infusing lipid directly into the distal part of the small intestine, alters gastrointestinal (GI) motility and inhibits food intake. The ileal brake effect on eating behavior of the other macronutrients is currently unknown.
Objective:
The objective of this study was to investigate the effects of ileal infusion of sucrose and casein on food intake, release of GI peptides, gastric emptying rate and small-bowel transit time with safflower oil as positive control.
Design:
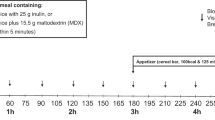
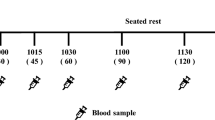
This randomized, single-blind, crossover study was performed in 13 healthy subjects (6 male; mean age 26.4±2.9 years; mean body mass index 22.8±0.4 kg m−2) who were intubated with a naso–ileal catheter. Thirty minutes after the intake of a standardized breakfast, participants received an ileal infusion, containing control ((C) saline), safflower oil ((HL) 51.7 kcal), low-dose casein ((LP) 17.2 kcal) or high-dose casein ((HP) 51.7 kcal), low-dose sucrose ((LC) 17.2 kcal) and high-dose sucrose ((HC) 51.7 kcal), over a period of 90 min. Food intake was determined during an ad libitum meal. Visual analogue score questionnaires for hunger and satiety and blood samples were collected at regular intervals.
Results:
Ileal infusion of lipid, protein and carbohydrate resulted in a significant reduction in food intake compared with control (HL: 464.3±90.7 kcal, P<0.001; HP: 458.0±78.6 kcal, P<0.005; HC: 399.0±57.0 kcal, P<0.0001 vs control: 586.7±70.2 kcal, P<0.001, respectively). A reduction in energy intake was still apparent when the caloric amount of infused nutrients was added to the amount eaten during the ad libitum meal.
Secretion of cholecystokinin and peptide YY but not of glucagon-like peptide-1 (7–36) was increased during ileal perfusion of fat, carbohydrates and protein. During ileal perfusion of all macronutrients, a delay in gastric emptying and intestinal transit was observed, but differences were not significant compared with control.
Conclusion:
Apart from lipids, also sucrose and casein reduce food intake on ileal infusion, thereby activating the ileal brake. In addition to food intake, also satiety and GI peptide secretion were affected.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
World Health Organization. Factsheet 311: Obesity and Overweight. Updated March 2013. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/.
Finkelstein EA, Trogdon JG, Cohen JW, Dietz W . Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009; 28: w822–w831.
Maljaars PW, Peters HP, Mela DJ, Masclee AA . Ileal brake: a sensible food target for appetite control. A review. Physiol Behav 2008; 95: 271–281.
Shin HS, Ingram JR, McGill AT, Poppitt SD . Lipids, CHOs, proteins: can all macronutrients put a 'brake' on eating? Physiol Behav 2013; 120: 114–123.
Koopmans HS, Sclafani A . Control of body weight by lower gut signals. Int J Obes 1981; 5: 491–495.
Atkinson RL, Whipple JH, Atkinson SH, Stewart CC . Role of the small bowel in regulating food intake in rats. Am J Physiol 1982; 242: R429–R433.
Welch I, Saunders K, Read NW . Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology 1985; 89: 1293–1297.
Welch IM, Sepple CP, Read NW . Comparisons of the effects on satiety and eating behaviour of infusion of lipid into the different regions of the small intestine. Gut 1988; 29: 306–311.
Maljaars J, Romeyn EA, Haddeman E, Peters HP, Masclee AA . Effect of fat saturation on satiety, hormone release, and food intake. Am J Clin Nutr 2009; 89: 1019–1024.
Maljaars PW, Peters HP, Kodde A, Geraedts M, Troost FJ, Haddeman E et al. Length and site of the small intestine exposed to fat influences hunger and food intake. Br J Nutr 2011; 106: 1609–1615.
Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA . Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes 2008; 32: 1633–1639.
Maljaars PW, van der Wal RJ, Wiersma T, Peters HP, Haddeman E, Masclee AA . The effect of lipid droplet size on satiety and peptide secretion is intestinal site-specific. Clin Nutr 2012; 31: 535–542.
Jain NK, Boivin M, Zinsmeister AR, Brown ML, Malagelada JR, DiMagno EP . Effect of ileal perfusion of carbohydrates and amylase inhibitor on gastrointestinal hormones and emptying. Gastroenterology 1989; 96: 377–387.
Layer P, Holst JJ, Grandt D, Goebell H . Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci 1995; 40: 1074–1082.
Layer P, Peschel S, Schlesinger T, Goebell H . Human pancreatic secretion and intestinal motility: effects of ileal nutrient perfusion. Am J Physiol 1990; 258: G196–G201.
Chaikomin R, Wu KL, Doran S, Meyer JH, Jones KL, Feinle-Bisset C et al. Effects of mid-jejunal compared to duodenal glucose infusion on peptide hormone release and appetite in healthy men. Regul Pept 2008; 150: 38–42.
Lavin JH, Wittert GA, Andrews J, Yeap B, Wishart JM, Morris HA et al. Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am J Clin Nutr 1998; 68: 591–598.
Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab 2007; 293: E743–E753.
Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 2005; 82: 41–48.
Read NW, McFarlane A, Kinsman RI, Bates TE, Blackhall NW, Farrar GB et al. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology 1984; 86: 274–280.
Geraedts MC, Troost FJ, Munsters MJ, Stegen JH, de Ridder RJ, Conchillo JM et al. Intraduodenal administration of intact pea protein effectively reduces food intake in both lean and obese male subjects. PLoS One 2011; 6: e24878.
Ryan AT, Luscombe-Marsh ND, Saies AA, Little TJ, Standfield S, Horowitz M et al. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 2013; 98: 300–311.
Van Strien T, Rookus MA, Bergers GP, Frijters JE, Defares PB . Life events, emotional eating and change in body mass index. Int J Obes 1986; 10: 29–35.
Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB . Further characterisation of the 'ileal brake' reflex in man—effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut 1988; 29: 1042–1051.
Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993; 104: 1640–1647.
Ledeboer M, Masclee AA, Jansen JB, Lamers CB . Effect of equimolar amounts of long-chain triglycerides and medium-chain triglycerides on small-bowel transit time in humans. JPEN J Parenter Enteral Nutr 1995; 19: 5–8.
Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM . Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr 2004; 58: 212–218.
Geraedts MC, Takahashi T, Vigues S, Markwardt ML, Nkobena A, Cockerham RE et al. Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab 2012; 303: E464–E474.
Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012; 61: 187–196.
Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B . The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 2013; 10: 729–740.
Rolls BJ, Kim S, McNelis AL, Fischman MW, Foltin RW, Moran TH . Time course of effects of preloads high in fat or carbohydrate on food intake and hunger ratings in humans. Am J Physiol 1991; 260: R756–R763.
Cook CG, Andrews JM, Jones KL, Wittert GA, Chapman IM, Morley JE et al. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am J Physiol 1997; 273: R755–R761.
Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M et al. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 2012; 96: 474–482.
Hill JO, Wyatt HR, Reed GW, Peters JC . Obesity and the environment: where do we go from here? Science 2003; 299: 853–855.
Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M . Protein, weight management, and satiety. Am J Clin Nutr 2008; 87: 1558S–1561SS.
Mellinkoff SM, Frankland M, Boyle D, Greipel M . Relationship between serum amino acid concentration and fluctuations in appetite. J Appl Physiol 1956; 8: 535–538.
Cummings DE, Overduin J . Gastrointestinal regulation of food intake. J Clin Invest 2007; 117: 13–23.
Sjolund K, Sanden G, Hakanson R, Sundler F . Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 1983; 85: 1120–1130.
Geraedts MC, Troost FJ, Tinnemans R, Soderholm JD, Brummer RJ, Saris WH . Release of satiety hormones in response to specific dietary proteins is different between human and murine small intestinal mucosa. Ann Nutr Metab 2010; 56: 308–313.
Keller J, Holst JJ, Layer P . Inhibition of human pancreatic and biliary output but not intestinal motility by physiological intraileal lipid loads. Am J Physiol Gastrointest Liver Physiol 2006; 290: G704–G709.
Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology 1993; 105: 733–739.
Maljaars PW . Intestinal fat and eating behavior: role of the ileal brake, chapter 7:both intestinal site and timing of fat delivery affect appetite in humans. PhD thesis (Unpublished) 2010; 109–126.
Duca FA, Swartz TD, Sakar Y, Covasa M . Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes 2013; 37: 375–381.
Stewart JE, Seimon RV, Otto B, Keast RS, Clifton PM, Feinle-Bisset C . Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr 2011; 93: 703–711.
Chen DC, Stern JS, Atkinson RL . Effects of ileal transposition on food intake, dietary preference, and weight gain in Zucker obese rats. Am J Physiol 1990; 258: R269–R273.
Acknowledgements
The research was funded by TI Food and Nutrition, a public-private partnership on pre-competitive research in food and nutrition. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author Contributions
FJT, HFH and AAMM designed the research; MvA and FJT conducted the research; MvA and DR analyzed data and performed the statistical analyses; DR performed the hormone analyses; MvA, FJT, HFH and AAMM contributed to interpretation of the results; MvA, FJT and AAMM wrote the manuscript; and AAMM had primary responsibility for the final content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
van Avesaat, M., Troost, F., Ripken, D. et al. Ileal brake activation: macronutrient-specific effects on eating behavior?. Int J Obes 39, 235–243 (2015). https://doi.org/10.1038/ijo.2014.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.112
This article is cited by
-
Small intestinal CaSR-dependent and CaSR-independent protein sensing regulates feeding and glucose tolerance in rats
Nature Metabolism (2024)
-
Interplay between grain digestion and fibre in relation to gastro-small-intestinal passage rate and feed intake in pigs
European Journal of Nutrition (2021)
-
Effect of bolus enteral tube feeding on body weight in ambulatory adults with obesity and type 2 diabetes: a feasibility pilot randomized trial
Nutrition & Diabetes (2020)
-
Ileal Transposition in Rats Reduces Energy Intake, Body Weight, and Body Fat Most Efficaciously When Ingesting a High-Protein Diet
Obesity Surgery (2020)
-
Effects of lipid emulsion particle size on satiety and energy intake: a randomised cross-over trial
European Journal of Clinical Nutrition (2018)