Abstract
Recently, cardio–renal interactions have been considered to be important and it has been demonstrated that mild renal dysfunction is associated with left ventricular hypertrophy (LVH). However, the correlation between LVH and subclinical renal damage is unclear. We investigated this association by assessing pretransplant biopsies from living kidney donors with normal renal function. We retrospectively categorized 238 living kidney donors into tertiles according to the percentage of global glomerulosclerosis (%GGS) observed in pretransplant biopsies (low, 0–3.45% (n=80); moderate, 3.46–11.76% (n=78); high, ⩾11.77% (n=80)) to analyze trends in their left ventricular mass index (LVMI) measured by echocardiography and baseline factors. LVH was defined as LVMI >110 g m−2 in female and >125 g m−2 in male subjects. We used a logistic regression model to evaluate any correlations between %GGS and LVH. LVMI increased significantly with increasing tertiles of %GGS, as did the prevalence of left ventricular remodeling and LVH. According to multivariate logistic regression analysis, subjects with high %GGS tertiles had a sevenfold greater risk of LVH than did those with low tertiles, even after adjusting for age, sex, systolic blood pressure, history of diabetes mellitus, total serum cholesterol and glomerular filtration rate (GFR) measured by a radioisotopic technique. There is an association between GGS and LVH in subjects with normal renal function. This association is significant after adjustment for age, sex, blood pressure, GFR and other atherogenic factors.
Similar content being viewed by others
Introduction
Recently, basic and clinical research have improved our understanding of cardio–renal damage and suggested that an interaction between pathophysiological (that is, hemodynamic, inflammatory and neuro-hormonal) mechanisms amplifies structural and functional cardio–renal derangement.1 The presence of both renal dysfunction and left ventricular hypertrophy (LVH) indicates cardiovascular risk and a poorer prognosis compare with the isolated cardiac or renal damage. This interaction is evident even in patients with mild renal insufficiency.2 According to the Multi-Ethnic Study of Atherosclerosis, concentrations of cystatin C, a superior marker of renal function, were inversely associated with left ventricular end-diastolic volumes and directly associated with concentricity independent of traditional cardiovascular risk factors in 4910 subjects with normal to mild renal dysfunction.3 In addition, Fesler et al.4 reported that increased left ventricular mass predicts subsequent renal dysfunction in both chronic kidney disease (CKD) patients and healthy subjects. However, it remains unclear whether subclinical renal damage, such as that detectable only by histological examination, is associated with altered left ventricular geometry in healthy subjects.
Although in general living kidney donors are healthy individuals, their pretransplant biopsy (0-h biopsy) specimens, obtained immediately after nephrectomy, often reveal varying degrees of global glomerulosclerosis (GGS) and arteriolosclerosis. In the general population, GGS is reportedly present in 1–3% of total glomeruli in adult subjects older than 50 years of age: the number of subjects with GGS increases with increasing age, reaching up to 30% by 80 years of age.5, 6 However, the precise causes are still unknown. A study analyzing biopsy specimens from 1208 living kidney donors reported that GGS is not completely attributable to age-related decline in glomerular filtration rate (GFR).7 On the other hand, we have noted that preoperative echocardiography in these subjects occasionally reveals LVH or left ventricular remodeling. LVH and left ventricular remodeling are common in hypertensive subjects and the elderly;8 however, some of our subjects with LVH have been normotensive and not elderly.
Therefore, we examined the cardio–renal interaction relationship between GGS at 0-h biopsies and LVH in near healthy subjects.
Materials and Methods
Patients
Three hundred and nine consecutive living kidney donors from March 2006 to October 2012 were initially included. Twenty-two of these donors were excluded because a 0-h biopsy or preoperative echocardiography was not performed. Thirty-nine donors were excluded because of inadequate biopsy specimens that contained fewer than 10 glomeruli or included simple cysts. Another 10 donors were excluded because some relevant laboratory findings were missing. The remaining 238 subjects were enrolled in this study. No subject had a history of stroke, coronary heart disease or heart failure. This study was part of a retrospective cohort study conducted by our department, which was approved by the Clinical Research Ethical Committee of the Kyushu University of Japan (Practice #24–54). Written informed consent was obtained from each subject.
Evaluation of echocardiography
Echocardiographic studies had been performed within the 6 months before donor nephrectomy using a iE33 echography system (Philips Health Care, Andover, MA, USA), Vivid 7 Dimension cardiovascular ultrasound system (GE Healthcare, Chalfont St Giles, Bucks, UK) or Artida 4D (Toshiba Medical Systems, Tochigi, Japan). All tracings were evaluated by several laboratory technicians and cardiologists. Overall, one-dimensional left ventricular measurements and two-dimensional (apical 4- and 2-chamber) views were obtained, in accordance with the recommendations of the American Society of Echocardiography. Relative wall thickness at end diastole was calculated as the ratio of the sum of left ventricular septal and posterior wall thickness to left ventricular internal end-diastolic diameter. Left ventricular mass was calculated using the Penn-cube method as described by Devereux et al.9 LVH was defined as left ventricular mass index greater than 110 g m−2 in female and 125 g m−2 in male subjects.10 Left ventricular remodeling was defined as increased relative wall thickness (more than 0.45) without hypertrophy. Concentric and eccentric LVH were defined as the presence or absence of increased relative wall thickness (more than 0.45), respectively.
Definitions of renal function and other relevant clinical variables before surgery
Hypertension was considered to be present if at least two consecutive resting blood pressure measurements before surgery met the criteria of the Seventh Joint National Committee Guidelines on Prevention, Detection, Evaluation and Treatment of High Blood Pressure11 or the subject had an established history of hypertension that had necessitated medical treatment. GFR was determined based on urinary clearance of 99mtechnetium-labeled diethylene triamine pentaacetic acid using the constant infusion technique with urine collection and normalized according to body surface area. Diabetes mellitus was defined as a fasting blood glucose concentration of more than 126 mg dl−1 and hemoglobin A1c more than 6.5% during preoperative evaluation. Hemoglobin A1c was measured according to Japanese Diabetes Society/Japanese Society of Clinical Chemistry guidelines, which were standardized by adding 0.4% to the estimate as the National Glycohemoglobin Standardization Program equivalent value.12 All subjects who were defined as having impaired glucose tolerance or diabetes mellitus received strict dietary therapy or hypoglycemic agents or both until normal glucose tolerance had been achieved. By the time of kidney donation, glucose tolerance had normalized in all donors. Obesity was defined as body mass index ⩾25 kg m−2. Dyslipidemia was defined as fasting total cholesterol concentration ⩾220 mg dl−1, triglyceride concentration ⩾150 mg dl−1 or use of lipid-modifying agents.
Data collection
Patient history, physical examination findings and laboratory values were collected. The assessed clinical variables were age, sex, comorbid medical conditions, use of medication (for example, antihypertensive drugs), body mass index and systolic and diastolic blood pressure. Body surface area was calculated by the du Bois method.13 Baseline laboratory data, such as blood hemoglobin and urea nitrogen, and serum creatinine, uric acid, total cholesterol, triglyceride and glucose concentrations, were obtained within a few days before surgery, along with the evaluation of a spot urine sample to assess the urinary protein/creatinine ratio and a dipstick urinary test.
Histological evaluation of 0-h renal biopsy
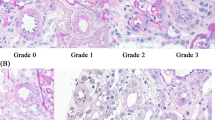
A wedge biopsy had been obtained from the outer cortex of each donor kidney immediately before transplantation. The sample was fixed in a paraformaldehyde–glutaraldehyde solution and then embedded in paraffin. Next, the sample was stained with hematoxylin-eosin, periodic acid-Schiff, methenamine silver and Masson trichrome stains. %GGS was determined using light microscopy. To quantify other characteristics of nephrosclerosis, such as renal arteriosclerosis, arteriolar hyalinosis and interstitial fibrosis, a semi-quantitative grading score based on the Banff criteria was used.14 Arteriolosclerosis was defined as follows:+1, fibro-intimal thickening<intimal diameter;+2, fibro-intimal thickening=intimal diameter; and+3, fibro-intimal thickening > intimal diameter. Each biopsy specimen was evaluated by at least two specialists in renal pathology (AT and KM) who were blinded to the clinical data.
Statistical analysis
Values are expressed as mean (s.d.) or number (%). The subjects were divided into tertiles according to %GGS (low, 0–3.45%; moderate, 3.46–11.76%; and high, ⩾11.77%). Trends in continuous and categorical values across the tertiles were examined by the Jonckheere–Terpstra and Cochran–Armitage tests, respectively.15, 16 The prevalence of left ventricular morphological changes and the various renal histology grades in each tertile of %GGS were evaluated using the linear regression analysis.17 After adjusting for the potential confounding risk factors (except triglyceride) that had been found to be statistically significant by the trend analyses (two-tailed P-value<0.05), the odds ratios and 95% confidence intervals for LVH were calculated by logistic regression. P<0.05 was considered statistically significant. All statistical analyses were conducted using JMP version 9.0.2, or SAS version 9.2 (SAS Institute, Cary, NC, USA) for trend testing of continuous values.
Results
Trend analysis of baseline characteristics
Table 1 shows the characteristics of all subjects according to each tertile of %GGS. Overall subjects, the mean number of glomeruli per biopsy was 26±13. Trace proteinuria (±) was detected by the dipstick urinary test in only two subjects; however, their urinary protein/creatinine ratios were less than 0.10 g per gCr. The other 236 subjects had negative dipstick urinary tests and urinary protein/creatinine ratios. The mean GFR was 117±27 ml min−1 per 1.73 m2. On admission, 28 subjects were taking antihypertensive drugs, which were angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in 17 cases. Eighteen subjects with dyslipidemia were receiving statins, bezafibrate or ezetimibe. Sixteen subjects had been diagnosed with diabetes mellitus during preoperative evaluation. Some of them had been receiving diet therapy and short-term antidiabetic medication or both to normalize their fasting blood glucose concentrations by the time of kidney donation. There were significant trends toward correlations between %GGS tertiles and age, hypertension, history of diabetes mellitus, dyslipidemia, systolic/diastolic blood pressure, pulse pressure, GFR, total serum cholesterol and triglyceride.
Distribution of left ventricular geometric changes according to tertiles of %GGS
Figure 1 shows the left ventricular morphological changes for each tertile of %GGS. The number of patients with left ventricular morphological changes was proportional to these tertiles (P<0.01). In addition, a substantial trend was observed between tertiles of %GGS and left ventricular mass index (P for this trend <0.01).
The distribution of left ventricular geometric changes and left ventricular mass index (LVMI). The overall prevalence of left ventricular remodeling and left ventricular hypertrophy (concentric and eccentric) is increased along with the tertiles of %GGS (P<0.01). A substantial trend is also observed between the tertiles of %GGS and LVMI (P for trend <0.01). %GGS; percentage of global glomerulosclerosis, LVMI; left ventricular mass index.
Histological analysis of the relationship between %GGS and other nephrosclerotic changes
Table 2 shows proportion of other histological findings characteristic of nephrosclerosis categorized according to Banff’s criteria. Individuals in the higher two categories of tertiles had a significantly higher proportion of advanced lesions than those in the lowest tertile.
Multivariate logistic regression models for LVH
The results of multivariate logistic regression analyses are shown in Table 3. In Model 1, unadjusted analysis showed that the OR for the highest tertile of %GGS was significantly greater than that for the lowest tertile. In Model 2, the OR for the highest tertile of %GGS remained significantly increased after adjusting for age and sex. Finally, even after adjusting for age, sex, systolic blood pressure, history of diabetes mellitus, total serum cholesterol and GFR, the subjects in the highest tertile of %GGS had a sevenfold greater risk of LVH than did those in the lowest tertile of %GGS (in Model 3, P<0.05).
Discussion
In the present study, we identified a significant association between GGS at 0-h biopsies and LVH as evaluated by preoperative echocardiography. This is the first study that has demonstrated a subclinical cardio–renal interaction in a near healthy population.
Nephrosclerosis is defined by the presence of glomerulosclerosis, arteriosclerosis, tubular atrophy and interstitial fibrosis on renal biopsy. Age or variations in blood pressure cannot completely explain the prevalence of GGS at 0-h biopsies from living kidney donors.5 In experimental models of nephrosclerosis, the renin–angiotensin–aldosterone system, sympathetic nerve overactivity, inflammatory reactions induced by reactive oxygen species and impaired bioavailability of nitric oxygen are considered to have important roles in the development of nephrosclerosis.18, 19, 20, 21
On the other hand, LVH is an adaptive response to increased cardiac work, which is the product of left ventricular pressure and stroke volume.22 The causes for this compensatory mechanism are the same as those listed above for nephrosclerosis.23, 24, 25
In patients with essential hypertension, LVH is more frequent in those with mild to moderate CKD than in those with normal renal function. Nardi et al.26 reported an increasingly higher prevalence of LVH with declining renal function in their study of 293 CKD patients. Moreover, after adjusting for age, sex, diastolic blood pressure and hemoglobin, they found that CKD is an independent predictor of left ventricular mass index. In the present study, we also determined that even in patients with subclinical kidney dysfunction, the relationship between LVH and renal histological damage is independent of other established factors for atherosclerosis. These findings suggest that nonclassical atherogenic factors such as the renin–angiotensin–aldosterone system in the peripheral organs, sympathetic nervous system overactivity, local inflammation and oxidative stress contribute to both left ventricular remodeling and chronic renal injury, even in subjects without CKD.27
Recently, an in vivo study showed that a decrease in nephron numbers is a significant determinant of increased blood pressure and cardio–renal damage generally, and of renal structural damage and LVH in particular.28, 29 Genetic or environmental factor may affect the degree of cardio–renal injury in healthy individuals.
Age is the most important factor in the relationship between nephrosclerosis and LVH. Age-related renal change is associated with the declining renal blood flow as shown by the xenon washout technique.30 Its blunted vasodilatory response to intrarenal acetylcholine administration suggests that age-related decline in renal blood flow has a vascular cause. Conversely, left ventricular dysfunction is sensed by baroreceptors, which activate the sympathetic nervous system. Impaired cardiovascular hemodynamic variables, such as increased end-diastolic pressure, increased peripheral vessel resistance and decreased stroke volume, potentially impair the peripheral blood flow in the kidneys.1 Although aging is the strongest risk factor for LVH, we speculate that left ventricular remodeling and arterial stiffness accelerate the age-related decline in renal blood flow and that this interaction produces a vicious cycle. Age-dependent GFR decreases are reportedly more pronounced in individuals with concentric LVH than in those with hypertension and normal left ventricular mass.4, 31 In addition, we identified by univariate analysis that pulse pressure increases along with tertiles of %GGS and found a weak correlation between left ventricular mass index and pulse pressure (Supplementary Figure 1). On the other hand, the association between decreased numbers of intact glomeruli and LVH may indicate that sodium retention contributes to these pathophysiological phenomena.32
This study has several limitations. First, there is a selection bias toward subjects with a family history of end-stage renal disease. The causes of renal diseases include hypertension and diabetes mellitus. Moreover, the subjects were self-selected by their agreement to serve as living kidney donors. Second, the biopsy specimens included only the superficial renal cortex. In the present study, the frequency of GGS was relatively higher than that reported by an autopsy study.5 However, according to Ito33, in the early stages of hypertension, renal injury occurs predominantly in the juxtamedullary nephrons, the majority of other nephrons remaining relatively intact. On the other hand, in an autopsy study, Kasiske34 reported that GGS occurs uniformly across all zones of the cortex. We could not confirm that the %GGS in our biopsy specimens is representative of the whole kidney. Finally, because this study is cross-sectional in nature, we cannot reach cause-and-effect conclusions. Further prospective studies are needed to clarify the long-term prognostic significance of the relationship between LVH and GGS.
Conclusion
We have demonstrated a correlation between the GGS and LVH in subjects with normal renal function. This relationship is independent of age, sex, blood pressure, renal function and other conventionally recognized atherogenic factors. A cardio–renal interaction may exist before there is an obvious decline in renal function.
References
Tsioufis C, Kokkinos P, Macmanus C, Thomopoulos C, Faselis C, Doumas M, Stefanadis C, Papademetriou V . Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypertens 2010; 28: 2299–2308.
Leoncini G, Viazzi F, Parodi D, Vettoretti S, Ratto E, Ravera M, Tomolillo C, Del Sette M, Bezante GP, Deferrari G, Pontremoli R . Mild renal dysfunction and subclinical cardiovascular damage in primary hypertension. Hypertension 2003; 42: 14–18.
Agarwal S, Thohan V, Shlipak MG, Lima J, Bluemke DA, Siscovick D, Gomes A, Herrington DM . Association between Cystatin C and MRI measures of left ventricular structure and function: Multi Ethnic study of atherosclerosis. Int J Nephrol 2011; 2011: 153868.
Fesler P, Du Cailar G, Ribstein J, Mimran A . Left ventricular remodeling and renal function in never-treated essential hypertension. J Am Soc Nephrol 2003; 14: 881–887.
Fogo AB . Mechanisms in nephrosclerosis and hypertension-beyond hemodynamics. J Nephrol 2001; 14 (Suppl 4): S63–S69.
Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Katafuchi R, Hirakata H, Okuda S, Tsuneyoshi M, Sueishi K, Fujishima M, Iida M . Risk factors for renal glomerular and vascular changes in an autopsy-based population survey: The Hisayama Study. Kidney Int 2003; 63: 1508–1515.
Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD . The Association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010; 152: 561–567.
Ribstein J, Du Cailar G, Zanchetti A . Cardiac and renal damage in the elderly hypertensive. J Renin Angiotensin Aldosterone Syst 2002; 3 (Suppl 1): S16–S24.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N . Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458.
Mancia G, De Baker G, Dominiczak A, Cifkova R, Fagard R, Germanò G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosion E, Fagard R, Lindholm LH, Manolis A, Nilsson PM, Redon J, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL . 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187.
Muntner P, Krousel-Wood M, Hyre AD, Stanley E, Cushman WC, Cutler JA, Piller LB, Goforth GA, Whelton PK . Antihypertensive prescriptions for newly treated patients before and after the main antihypertensive and lipid-lowering treatment to prevent heart attack trial results and seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure guidelines. Hypertension 2009; 53: 617–623.
Committee of Japanese diabetes society on the diagnosis criteria of diabetes mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 2010; 53: 450–467.
Du Bois D, Du Bois EF . A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 17: 863–871.
Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y . The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723.
Li Q, Li Z, Zheng G, Gao G, Yu K . Rank-Based Robust Tests for Quantitative-Trait Genetic Association Studies. Genet Epidemiol 2013; 37: 358–365.
Zheng G, Yang Y, Zhu X, Elston RC . Analysis of Genetic Association Studies. Gail M, Krickeberg K, Samet J, Tsiatis A, Wong W, (eds).. Statistics for Biology and Health. Springer: New York, NY, USA. 2012 PP 61–93.
Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, Tsuruya K, Iida M, Kiyohara Y, Sueishi K . Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from japanese elders: The Hisayama Study. Am J Kidney Dis 2010; 55: 21–30.
Remuzzi G, Perico N, Macia M, Ruggenenti P . The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl 2005; 99: S57–S65.
Chen Y, Lipkowitz MS, Salem RM, Fung MM, Bhatnagar V, Mahata M, Nievergelt CM, Rao F, Mahata SK, Schork NJ, Hicks PJ, Bowden DW, Freedman BI, Brophy VH, O'Connor DT, AASK. Progression of chronic kidney disease: Adrenergic genetic influence on glomerular filtration rate decline in hypertensive nephrosclerosis. Am J Nephrol 2010; 32: 23–30.
Imakiire T, Kikuchi Y, Yamada M, Kushiyama T, Higashi K, Hyodo N, Yamamoto K, Oda T, Suzuki S, Miura S . Effects of renin-angiotensin system blockade on macrophage infiltration in patients with hypertensive nephrosclerosis. Hypertens Res 2007; 30: 635–642.
Ono H, Ono Y, Takanohashi A, Matsuoka H, Frohlich ED . Apoptosis and glomerular injury after prolonged nitric oxide synthase inhibition in spontaneously hypertensive rats. Hypertension 2001; 38: 1300–1306.
London GM . Left ventricular hypertrophy: why does it happen? Nephrol Dial Transplant 2003; 18 (Suppl 8): viii2–viii6.
Pedrinelli R, Dell’Omo G, Di Bello V, Pellegrini G, Pucci L, Del Prato S, Penno G . Low-grade inflammation and microalbuminuria in hypertension. Arterioscler Thromb Vasc Biol 2004; 24: 2414–2419.
Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH . Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation 2003; 108: 1831–1838.
Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD . Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 2003; 108: 560–565.
Nardi E, Palermo A, Mulè G, Cusimano P, Cottone S, Cerasola G . Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens 2009; 27: 633–641.
Chade AR, Lerman A, Lerman LO . Kidney in early atherosclerosis. Hypertension 2005; 45: 1042–1049.
Rothermund L, Lorenz M, Schnieber A, Eberson J, Bauhaus I, Bernhard Haug M, Schulz A, Keller F, Vetter R, Kreutz R . Impact of nephron number dosing on cardiorenal damage and effects of ACE inhibition. Am J Hypertens 2010; 24: 474–481.
Singh RR, Denton KM, Bertram JF, Jefferies AJ, Head GA, Lombardo P, Schneider-Kolsky M, Moritz KM . Development of cardiovascular disease due to renal insufficiency in male sheep following fetal unilateral nephrectomy. J Hypertens 2009; 27: 386–396.
Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, Merrill JP . Senescence and the renal vasculature in normal man. Circ Res 1974; 34: 309–316.
Watanabe S, Okura T, Liu J, Miyoshi K, Fukuoka T, Hiwada K, Higaki J . Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res 2003; 26: 895–899.
Perticone F, Maio R, Ruberto C, Cassano S, Tripepi G, Perticone M, Sesti G, Zoccali C . Kidney function and risk factors for left ventricular hypertrophy in untreated uncomplicated essential hypertension. Am J Kidney Dis 2008; 52: 74–84.
Ito S . Cardiorenal connection in chronic kidney disease. Clin Exp Nephrol 2012; 16: 8–16.
Kasiske BL . Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 1987; 31: 1153–1159.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Haruyama, N., Tsuchimoto, A., Masutani, K. et al. Subclinical nephrosclerosis is linked to left ventricular hypertrophy independent of classical atherogenic factors. Hypertens Res 37, 472–477 (2014). https://doi.org/10.1038/hr.2013.154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2013.154
Keywords
This article is cited by
-
Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease
Nature Reviews Nephrology (2023)
-
Malnutrition, renal dysfunction and left ventricular hypertrophy synergistically increase the long-term incidence of cardiovascular events
Hypertension Research (2016)
-
Plasma aldosterone and its relationship with left ventricular mass in hypertensive patients with early-stage chronic kidney disease
Hypertension Research (2015)