Abstract
Implantation of bone marrow-derived mononuclear cells (BMMCs) is known to accelerate blood flow recovery in a hindlimb ischemia model in mice. However, the neovascularization capacity of BMMCs from diabetic mice is impaired. Resveratrol, a natural polyphenolic compound abundant in red wine, is known to extend the lifespan of high cholesterol-fed mice. We tested whether resveratrol improves the neovascularization capacity of BMMCs from diabetic mice. Diabetes was induced by the injection of streptozotocin into C57B/6 mice. BMMCs from normal mice and diabetic mice were implanted into the ischemic limb induced by ligation of the unilateral femoral artery. Blood flow recovery measured by the laser Doppler method was significantly decreased in mice that received BMMCs from diabetic mice compared with BMMCs from normal mice. However, ex vivo treatment of BMMCs from diabetic mice, but not from normal mice, with resveratrol for 30 min significantly improved blood flow recovery. Capillary density measured by PECAM-1 positive cells was significantly increased in mice that received either normal BMMCs or diabetic BMMCs treated with resveratrol. Treatment of BMMCs from diabetic mice with resveratrol increased mRNA expression of vascular endothelial growth factor and endothelial nitric oxide synthase and decreased production of reactive oxygen species. Resveratrol improved the impaired neovascularization capacity of BMMCs derived from diabetic mice. The effects of resveratrol may be due to a reduction of oxidative stress and an induction of angiogenic factors. Resveratrol may be beneficial by improving the neovascularization capacity of BMMCs in patients with diabetes mellitus.
Similar content being viewed by others
Introduction
The ability of organisms to develop collateral vessels is an important protective mechanism against ischemia that is also important in wound repair. Initially, ischemia-induced postnatal neovascularization was thought to rely exclusively on sprouting, proliferation and migration of pre-existing mature endothelial cells, a process known as angiogenesis. However, Asahara et al.1 have shown that CD34-positive peripheral blood mononuclear cells have the ability to differentiate into mature endothelial cells, and evidence suggests that these cells, the so-called endothelial progenitor cells (EPCs), can home in on ischemic tissues and also contribute to postnatal neovascularization.2
Bone marrow-derived mononuclear cells (BMMCs) are the major source of EPCs. BMMCs can secrete various proangiogenic factors, such as vascular endothelial growth factor (VEGF) and angiopoietin-1, that are critical for the induction of angiogenesis.3 Autologous implantation of BMMCs improves blood flow recovery of patients with peripheral arterial occlusive diseases.4 Moreover, recent clinical trials have reported that implantation of BMMCs or EPCs improved cardiac function both in ischemic cardiomyopathy5 and after acute myocardial infarction.6 However, both positive and negative results of BMMCs therapy on cardiac function after acute myocardial infarction have been reported.7
Patients with peripheral arterial occlusive disease or ischemic heart disease frequently have other risk factors for atherosclerosis, such as diabetes mellitus, hypertension and hyperlipidemia, which reduce the number and function of EPCs.8 The number of EPCs is decreased in patients with type 1 diabetes,9 and Tamarat et al.10 have shown that the mobilization of BMMCs to the peripheral blood is significantly impaired in streptozotocin (STZ)-induced diabetic mice, resulting in an abrogated neovascularization after implantation into an ischemic hindlimb. Moreover, it has been reported that EPCs from patients with type 2 diabetes show impaired proliferation, adhesion and tube formation in a Matrigel assay.11
Resveratrol (trans-3,4,5′-trihydroxystilbene) is a natural polyphenolic compound abundant in grape skin and red wine. A moderate intake of red wine is associated with decreased incidence of ischemic heart disease despite a high-fat diet, a trend called the French paradox.12, 13 The precise mechanisms of cardiovascular protection by red wine are not completely understood but may be due, at least in part, to polyphenolic compounds such as resveratrol. For instance, resveratrol reduces the oxidation of low-density lipoproteins,14 inhibits the proliferation of vascular smooth muscle cells15 and protects against cigarette smoke-mediated oxidative stress,16 indicating the anti-atherogenic effects of resveratrol. Resveratrol also increases the number and activity of EPCs from human peripheral blood as well as the expression of VEGF,17 suggesting that resveratrol has angiogenic properties. However, it has also been shown that resveratrol inhibits VEGF and bFGF-induced angiogenesis.18, 19 These data suggest that the effect of resveratrol on angiogenesis may be context dependent.
Therefore, we investigated whether resveratrol could improve the neovascularization capacity of BMMCs from diabetic mice. In this study, we show that the short-term ex vivo treatment of BMMCs from diabetic mice with resveratrol improved impaired angiogenic activity.
Methods
Materials
Streptozotocin and resveratrol were purchased from Sigma Chemical Co. (St Louis, MO, USA). Anti-PECAM1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Dulbecco's modified Eagle's medium was purchased from GIBCO BRL (Carlsbad, CA, USA). Other chemical reagents were purchased from Wako Pure Chemicals (Osaka, Japan), unless specified otherwise.
Induction of diabetes
All procedures and animal care practices were approved by the Committee on Ethics of Animal Experiments, Kyushu University, and were conducted in accordance with Institutional Guidelines. C57BL/6 mice (4–5 weeks old, Kyudo Co. Ltd, Saga, Japan) were intraperitoneally injected with 40 mg kg−1 of STZ in 0.05 M sodium citrate, pH 4.5, daily for 5 days.20 Three days after the last injection, blood glucose levels were measured through the tail vein. If blood glucose was less than 162 mg per 100 ml, the mice received five additional injections of STZ at the same dosage. Mice with glucose levels more than 200 mg per 100 ml were considered to be diabetic. In the non-diabetic groups, mice were injected intraperitoneally with 0.05 M sodium citrate. BMMCs were prepared after waiting for another 4 weeks to establish the diabetic condition.
A murine model of hindlimb ischemia, isolation of BMMCs and implantation of BMMCs
Unilateral hindlimb ischemia was created in 10-week-old C57BL/6 mice to which the age of BMMC donor mice was matched.21 After the mice were given general anesthesia by ether, the left femoral artery was exposed and ligated, and its branches were dissected and excised. The femoral artery was excised from its proximal origin as a branch of the external iliac artery to the distal point where it bifurcates into the saphenous and popliteal arteries. BMMCs were isolated from the femur and tibiae of 10-week-old normal and diabetic C57BL/6 mice by flushing the bone marrow cavities followed by density gradient centrifugation of the marrow.22 Once isolated, BMMCs were incubated with resveratrol (10 and 100 μM) in phosphate-buffered saline (PBS) or PBS alone for 30 min. The doses of resveratrol were chosen on the basis of the activation of SIRT1, a longevity gene, by resveratrol.23 After centrifugation, the cells were washed once with PBS and resuspended in PBS. BMMCs (3 × 106) or PBS were injected at 2 points of the adductor muscle and at 1 point of the quadriceps and gastrocnemius muscles of the ischemic hindlimb of normal mice.
Experimental groups
The following four groups were examined: (1) hindlimb ischemia operation in normal mice followed by PBS injection into ischemic muscles (control; Con), (2) hindlimb ischemia operation in normal mice followed by implantation of BMMCs derived from normal mice into ischemic muscles (normal bone marrow; NB), (3) hindlimb ischemia operation in normal mice followed by implantation of untreated BMMCs derived from diabetic mice into ischemic muscles (diabetic bone marrow; DB) and (4) hindlimb ischemia operation in normal mice followed by implantation of resveratrol-treated (100 μM for 30 min) BMMCs derived from diabetic mice into ischemic muscles (DB+resveratrol; DBR).
Laser Doppler perfusion imaging
Laser Doppler perfusion imaging was performed at days 0, 4, 7 and 14 after surgery. Excess limb hair was removed by depilatory cream, and mice were placed on a heating plate at 37°C to minimize temperature variation. To reduce variations owing to ambient light, temperature and experimental procedures, blood flow was calculated in the foot and expressed as a ratio of ischemic to non-ischemic leg (Doppler ratio).
Capillary density
After 14 days of femoral artery ligation, all mice were killed. The gastrocnemius muscles of ischemic and non-ischemic limbs were retrieved, fixed in 10% buffered formalin, embedded in paraffin and sectioned at a thickness of 5 μm for immunohistochemistry. Blocking was performed with 3% fat-free milk for 1 h at room temperature. The section was incubated with an anti-mouse PECAM-1 goat monoclonal antibody (dilution 1:500) at 4°C followed by incubation with a rabbit anti-goat IgG antibody labeled by Alexa fluoro 555 (dilution 1:1000, Molecular Probes, Carlsbad, CA, USA). The sections were then rinsed in PBS three times and observed under a confocal microscope.
Capillary density was analyzed from 15 different fields in three sections of each mouse under microscopy ( × 200) and expressed as PECAM-1 positive cells/high power field.
Real-time PCR
Total mRNA was extracted from BMMCs of all groups with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (2 μg) was reverse transcribed to cDNA with oligo-dT using a reverse transcription kit (Promega, Madison, WI, USA). Real-time reverse transcription-PCR analysis (Applied Biosystems Inc., Foster City, CA, USA; ABI 7500) was carried out with 2 μl of cDNA template using a TaqMan probe (Applied Biosystems Inc.) and β-actin as a reference control. The sequences for sense, antisense and the specific fluorescent probe were as follows.
Endothelial nitric oxide synthase (eNOS)-sense: 5′-CAGGCATCACCAGGAAGAAGA-3′, eNOS-antisense: 5′-GGCCAGTCTCAGAGCCATACA-3′, eNOS-P: 5′-TTGCCTTCACACGCTTCGCC-3′, VEGF-sense: 5′-CCCACGACAGAAGGAGAGCA-3′, VEGF-antisense: 5′-GCACACAGGACGGCTTGAA-3′, VEGF-P: 5′–CTACTGCCGTCCGATTGAGACCC-3′, VEGF-R2 sense: 5′-GCTGTGAACGCTTGCCTTAT-3′, VEGF-R2 antisense: 5′-CATCTTGACGGCTACTGTTTTG-3′, VEGF-R2-P: 5′-CCAAAAGCGTCTGCCTCAATCAC-3′.
Measurement of reactive oxygen species production
The level of intracellular reactive oxygen species (ROS) was determined by measuring oxidative conversion of cell-permeable 2′,7′-dichlorofluorescein diacetate to fluorescent dichlorofluorescein on reaction with cellular oxidants.24 Briefly, after BMMCs were washed by cold PBS (pH 7.4), the cells were incubated with 10 μM 2′,7′-dichlorofluorescein diacetate (Beyotime Company, Haimen, China) in Dulbecco's modified Eagle's medium at 37°C for 30 min in the dark and then washed three times with cold PBS. The dichlorofluorescein fluorescence of 1 × 106 cells was detected by fluorospectrophotometer (970CRT V2.0, Shanghai, China) analysis at an excitation wavelength of 492 nm and an emission wavelength of 525 nm.
Measurement of malon dialdehyde level
Malon dialdehyde (MDA), one of the end products of lipid peroxidation, was measured by using MDA assay kits (Jiancheng Bioengineering Institute, Nanjing, China).25 MDA reacts with thiobarbituric acid to form a stable chromophoric production that can be detected by a spectrophotometer at a wavelength of 532 nm.26 Briefly, BMMCs (3 × 106) were washed twice in cold PBS and homogenized. The homogenate was centrifuged for 10 min at 10 000 r.p.m. at 4°C, and the supernatant was used for assays according to the manufacturer’s instructions. The level of MDA was normalized by the protein concentration of the BMMCs. The results were expressed as nmol MDA per mg protein.
Measurement of superoxide dismutase activity
Activity of superoxide dismutase (SOD) was determined by using the SOD assay kits (Jiancheng Bioengineering Institute). The assay for SOD activity was based on its ability to inhibit the oxidation of hydroxylamine by O2− produced from the xanthine–xanthine oxidase system.25 One unit of SOD activity was defined as the amount that reduced the absorbance at 550 nm by 50%. The supernatant of protein made as described earlier was used for SOD activity assays according to the manufacturer’s instructions. The activity of SOD was also normalized by protein concentration of BMMCs, and the results were expressed as units per mg protein. Protein content was measured using a bicinchoninic acid assay kit (Beyotime Company).
Statistical analysis
One-way analysis of variance with the Bonferroni post hoc test was used to test differences between the groups. P-values <0.05 were considered to be significant. The data are expressed as mean±s.e.m.
Results
Resveratrol increased the neovascularization capacity of BMMCs derived from diabetic mice
There were no significant differences in body weight, heart rate or systolic blood pressure among four groups before and after 14 days of femoral artery ligation and implantation of BMMCs (Table 1).
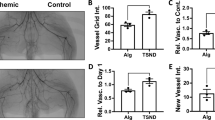
Figure 1a shows representative results of laser Doppler blood flow measurements at days 0, 4, 7 and 14 after femoral artery ligation. Blood flow recovery after hindlimb ischemia was significantly increased by implantation of BMMCs derived from normal mice (Figure 1b: 53.2±9.8% in Con vs. 81.5±3.8%, in NB, P<0.01) at day 14. The neovascularization capacity of BMMCs derived from diabetic mice was significantly impaired compared with that derived from normal C57BL/6 mice (69.2±15.5% in DB vs. 81.5±3.8% in NB, P<0.05), which was significantly improved by ex vivo treatment with resveratrol (93.2±6.1% in DBR vs. 69.2±15.5% in DB, P<0.01) at day 14.
Representative pictures of laser Doppler blood flow measurements and microphotographs of immunostaining of ischemic hindlimb tissue. (a) The left hindlimb was subjected to femoral artery ligation. Laser Doppler perfusion imaging was serially recorded at days 0, 4, 7 and 14 after ligation. The picture at day 0 was recorded immediately after femoral artery ligation. (b) The line graphs indicate the ratio of ischemic to normal limb blood flow. The ratio before femoral artery ligation was designated as 1.0. Data are mean±s.e.m. n=8 in control and NB groups, and n=5 in the diabetic mice (DM) and DMR groups. †P<0.05 NB vs. DB; *P<0.05 DBR vs. DB; **P<0.01 DBR vs. DB; #P<0.05 DBR vs. NB. (c) Microphotographs of immunostaining for PECAM-1. The number of PECAM-1-positive cells was counted in high power field. (d) Bar graphs indicate the number of PECAM-1-positive cells in high power field. Data are mean±s.e.m. n=8 in Con and NB groups, and n=5 in the DM and DMR groups. **P<0.01 vs. Con; #P<0.05 vs. NB; P<0.05 vs. DB. (e) A bar graph indicates the ratio of ischemic to normal limb blood flow in mice injected with phosphate-buffered saline (PBS) (Con), bone marrow-derived mononuclear cell (BMMCs) from normal mice (NB), and BMMCs from DB at 2 weeks. BMMCs were incubated with resveratrol (RV) for 30 min at concentrations indicated in the figure before injection into ischemic hindlimb. n=5–7, *P<0.05, **P<0.01 vs. Con.
We counted cells positive for PECAM-1, a specific marker of endothelium, in the ischemic hindlimb to compare capillary density at day 14 (Figure 1c). The implantation of normal BMMCs increased the capillary density, which was impaired in BMMCs derived from diabetic mice (Figure 1d). Incubation of BMMCs derived from diabetic mice with resveratrol restored neovascularization capacity to a level comparable to that of normal BMMCs.
At 2 weeks, low-dose resveratrol (10 μM) did not affect the angiogenic activity of BMMCs derived from both normal and diabetic mice (Figure 1e). Interestingly, high-dose resveratrol (100 μM) suppressed the angiogenic capacity of BMMCs derived from normal mice.
Resveratrol increased VEGF, VEGF receptor and eNOS expression in BMMCs
To clarify the mechanisms of the restoration of neovascularization capacity by resveratrol, we measured the mRNA levels of VEGF, its type 2 receptor (VEGF-R2) and eNOS (Figure 2). Expression levels of VEGF, VEGF-R2 and eNOS were suppressed in BMMCs derived from diabetic mice compared with those derived from normal mice. Resveratrol increased the expression of VEGF, VEGF-R2 and eNOS, indicating that the upregulation of these angiogenic factors may be responsible for the resveratrol-induced recovery of neovascularization capacity of BMMCs derived from diabetic mice.
Effect of resveratrol on gene expression in bone marrow-derived mononuclear cell (BMMCs). RNA from BMMCs derived from normal mice or from diabetic mice (DM) with or without resveratrol (RV) treatment (100 μM for 60 min) was prepared and analyzed by real-time PCR. Data are expressed as mean±s.e.m. **P<0.01 vs. control, ##P<0.01 vs. DM, n=6.
Resveratrol decreased oxidative stress in BMMCs derived from diabetic mice
It is generally accepted that oxidative stress is increased in the diabetic condition and that ROS downregulate angiogenic factors. Therefore, we examined the level of oxidative stress in BMMCs. SOD activity was decreased in BMMCs derived from diabetic mice, and it was restored by resveratrol treatment (Figure 3a). Levels of ROS and MDA, one of the end products of lipid peroxidation, were increased in BMMCs derived from diabetic mice (Figures 3b and c). The increased levels of ROS and MDA were normalized by resveratrol treatment. These data suggest that the anti-oxidative effect of resveratrol may play a critical role in the upregulation of angiogenic factors, which may be followed by restoration of the angiogenic capacity of BMMCs derived from diabetic mice.
Effects of resveratrol on oxidative stress in bone marrow-derived mononuclear cell (BMMCs). BMMCs derived from normal mice or from diabetic mice (DM) with or without resveratrol (RV) treatment (100 μM for 60 min) were examined for (a) superoxide dismutase (SOD) activity, (b) reactive oxygen species (ROS) production, and (c) malon dialdehyde (MDA) contents. Data are expressed as mean±s.e.m. **P<0.01 vs. control, ##P<0.01 vs. DM. n=7.
Discussion
In this study, we showed that brief ex vivo resveratrol treatment of BMMCs derived from diabetic mice improved their neovascularization capacity. Resveratrol treatment increased angiogenic activity, which was correlated with increased expression of VEGF and eNOS mRNA as well as increased SOD activity and which was inversely correlated with ROS production.
It is reported that hyperglycemia-induced ROS are responsible for impaired EPC function and angiogenesis. Ceradini et al.27 showed that transgenic overexpression of Mn-SOD or SOD mimetic manganese(III) meso-tetrakis(4-carboxyphenyl)porphyrin improved impaired ischemia-induced vasculogenesis in STZ-induced diabetic mice. Hyperglycemia-induced methylglyoxal modification of hypoxia-inducible factor-1 inhibited hypoxia-induced expression of stromal derived factor-1 and VEGF. Therefore, one possible mechanism by which resveratrol improved the impaired angiogenic activity of BMMCs derived from diabetic mice is through its anti-oxidant activity, as suggested in this and previous studies.14, 28 Reduction of ROS levels also increases nitric oxide bioavailability and improves angiogenesis. As eNOS mRNA expression was also increased by resveratrol, direct and indirect increases in nitric oxide bioavailability may be involved in the mechanisms behind resveratrol-enhanced angiogenesis. In addition, resveratrol is known to activate SIRT1,23 a longevity gene, and to prolong the lifespan of high-fat fed mice.29 SIRT1 is reported to enhance angiogenesis,30 and high medium glucose levels downregulate SIRT1 expression and EPC numbers in culture.31 Therefore, another possible mechanism is that resveratrol improved angiogenesis through activation of SIRT1.
The function of BMMCs is regulated by various angiogenesis-related factors, such as VEGF. VEGF induces growth and differentiation of EPCs, and also mobilizes and recruits EPCs from bone marrow into tissues.32 Furthermore, eNOS plays an important role in these processes as an essential mediator of the angiogenic property of VEGF. VEGF-induced endothelial cell migration and proliferation was strongly suppressed by inhibition of eNOS.33 Therefore, co-upregulation of VEGF and eNOS by resveratrol may be critical for the improvement of angiogenic activity.
It has also been reported that resveratrol promotes EPC proliferation, adhesion and migration, suggesting that resveratrol may promote angiogenesis.17 However, the effects of resveratrol on angiogenesis are controversial. In an experimental myocardial infarction in rats fed a high-fat diet, resveratrol increased capillary and arteriolar density in the peri-infarct area and improved left ventricular ejection fraction.34 By contrast, Lin et al.18 reported that resveratrol inhibited VEGF-induced migration and tube formation of human umbilical vein endothelial cells through inhibition of VEGF-induced Src kinase activation and peroxide production. Resveratrol also inhibited angiogenesis in chollioallantoic membranes and inhibited corneal neovascularization induced by VEGF or FGF-2.19 A recent study showed that red wine polyphenols are proangiogenic at low doses but antiangiogenic at high doses.35 The biphasic effect of polyphenols on angiogenesis may also explain the contradictory results of previous studies. The suppressive effects of high-dose resveratrol on the angiogenic capacity of normal BMMCs, compared with that of low-dose resveratrol, is consistent with the results of this study. However, the mechanism by which the biphasic effect was not observed in the angiogenic capacity of resveratrol-treated BMMCs derived from diabetic mice is not known. As ROS levels are higher in the diabetic condition, high-dose resveratrol may still adequately quench the ROS and restore the angiogenic capacity of BMMCs. However, ROS reduction by high-dose resveratrol in normal BMMCs may be excessive for an appropriate angiogenesis, resulting in a rather decreased angiogenic capacity. Although the mechanism is not clear, it may be possible that the brief ex vivo treatment of BMMCs with resveratrol used in this study may mimic the condition of systemic treatment with polyphenol at low doses. Further study is needed to clarify the precise mechanism by which such a brief treatment with resveratrol increased neovascularization capacity and the differential effects of resveratrol on BMMCs between diabetic and normal mice.
In addition, we could not exclude the possibility of the direct effect of resveratrol on angiogenesis that might be introduced along with BMMCs. We assume, however, that the direct effect of resveratrol may be minimal because we washed BMMCs with PBS after incubation with resveratrol.
A recent study showed that ex vivo pretreatment of BMMCs derived from patients with ischemic cardiomyopathy with NOS enhancer AVE9488 restored impaired neovascularization capacity through increased migration activity.36 The increased angiogenic activity of AVE9488-stimulated BMMCs was abrogated by a pharmacological eNOS inhibitor L-NAME, supporting the critical role of nitric oxide in BMMC-induced angiogenesis. This study and our data suggest that functional activities of BMMCs, in particular eNOS and nitric oxide bioavailability, may be critical for angiogenic activity after implantation in the ischemic hindlimb.
The limitation of this study is that we used normal mice as recipients of BMMC implantation. Therefore, it is not clear whether the brief treatment with BMMCs derived from diabetic mice with resveratrol effectively restores blood flow of ischemic hindlimbs in diabetic mice. Therefore, further study is necessary to apply autologous implantation of resveratrol-treated BMMCs to the treatment of peripheral artery diseases in the diabetic condition.
In conclusion, this study showed that brief ex vivo treatment of BMMCs derived from diabetic mice improved neovascularization capacity, possibly through the upregulation of eNOS and VEGF and suppression of oxidative stress. Resveratrol may provide a novel therapeutic application to cell therapy for peripheral artery diseases and ischemic heart diseases complicated by diabetes.
Conflict of interest
The authors declare no conflict of interest.
References
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM . Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–967.
Isner JM, Asahara T . Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest 1999; 103: 1231–1236.
Schatteman GC, Dunnwald M, Jiao C . Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol 2007; 292: H1–H18.
Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T . Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002; 360: 427–435.
Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FO, Assad JA, Carvalho AC, Branco RV, Rossi MI, Dohmann HJ, Willerson JT . Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation 2004; 110: II213–II218.
Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM . Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 2006; 355: 1222–1232.
Rosenzweig A . Cardiac cell therapy—mixed results from mixed cells. N Engl J Med 2006; 355: 1274–1277.
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S . Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001; 89: E1–E7.
Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ . Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004; 53: 195–199.
Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, Duriez M, Tobelem G, Levy BI . Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol 2004; 164: 457–466.
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC . Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002; 106: 2781–2786.
Gronbaek M, Deis A, Sorensen TI, Becker U, Schnohr P, Jensen G . Mortality associated with moderate intakes of wine, beer, or spirits. BMJ 1995; 310: 1165–1169.
Zern TL, Fernandez ML . Cardioprotective effects of dietary polyphenols. J Nutr 2005; 135: 2291–2294.
Frankel EN, Waterhouse AL, Kinsella JE . Inhibition of human LDL oxidation by resveratrol. Lancet 1993; 341: 1103–1104.
Poussier B, Cordova AC, Becquemin JP, Sumpio BE . Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg 2005; 42: 1190–1197.
Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I . Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2008; 294: L478–L488.
Wang XB, Huang J, Zou JG, Su EB, Shan QJ, Yang ZJ, Cao KJ . Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol 2007; 34: 1109–1115.
Lin MT, Yen ML, Lin CY, Kuo ML . Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol 2003; 64: 1029–1036.
Brakenhielm E, Cao R, Cao Y . Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J 2001; 15: 1798–1800.
Tanii M, Yonemitsu Y, Fujii T, Shikada Y, Kohno R, Onimaru M, Okano S, Inoue M, Hasegawa M, Onohara T, Maehara Y, Sueishi K . Diabetic microangiopathy in ischemic limb is a disease of disturbance of the platelet-derived growth factor-BB/protein kinase C axis but not of impaired expression of angiogenic factors. Circ Res 2006; 98: 55–62.
Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM . Mouse model of angiogenesis. Am J Pathol 1998; 152: 1667–1679.
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T . Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5: 434–438.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA . Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–196.
Ye J, Wang S, Leonard SS, Sun Y, Butterworth L, Antonini J, Ding M, Rojanasakul Y, Vallyathan V, Castranova V, Shi X . Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J Biol Chem 1999; 274: 34974–34980.
Li Y, Bao Y, Jiang B, Wang Z, Liu Y, Zhang C, An L . Catalpol protects primary cultured astrocytes from in vitro ischemia-induced damage. Int J Dev Neurosci 2008; 26: 309–317.
Ohkawa H, Ohishi N, Yagi K . Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358.
Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC . Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem 2008; 283: 10930–10938.
Miller NJ, Rice-Evans CA . Antioxidant activity of resveratrol in red wine. Clin Chem 1995; 41: 1789.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA . Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006; 444: 337–342.
Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S . SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 2007; 21: 2644–2658.
Balestrieri ML, Rienzo M, Felice F, Rossiello R, Grimaldi V, Milone L, Casamassimi A, Servillo L, Farzati B, Giovane A, Napoli C . High glucose downregulates endothelial progenitor cell number via SIRT1. Biochim Biophys Acta 2008; 1784: 936–945.
Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM . VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 1999; 18: 3964–3972.
Cai J, Jiang WG, Ahmed A, Boulton M . Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res 2006; 71: 20–31.
Penumathsa SV, Koneru S, Samuel SM, Maulik G, Bagchi D, Yet SF, Menon VP, Maulik N . Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic Biol Med 2008; 45: 1027–1034.
Baron-Menguy C, Bocquet A, Guihot AL, Chappard D, Amiot MJ, Andriantsitohaina R, Loufrani L, Henrion D . Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. FASEB J 2007; 21: 3511–3521.
Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, Rossig L, Koehl U, Koyanagi M, Mohamed A, Brandes RP, Martin H, Zeiher AM, Dimmeler S . Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA 2006; 103: 14537–14541.
Acknowledgements
This study was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (19590867) and Kimura Foundation Research Grant 2007 to TI. LG was supported by the Japan-China Sasakawa Medical Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gan, L., Matsuura, H., Ichiki, T. et al. Improvement of neovascularization capacity of bone marrow mononuclear cells from diabetic mice by ex vivo pretreatment with resveratrol. Hypertens Res 32, 542–547 (2009). https://doi.org/10.1038/hr.2009.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.67
Keywords
This article is cited by
-
Association of alcohol intake with risk of diabetic retinopathy: a meta-analysis of observational studies
Scientific Reports (2017)
-
Resveratrol in cardiovascular disease: what is known from current research?
Heart Failure Reviews (2012)