Abstract
Few therapies can reverse the proangiogenic activity of senescent mesenchymal stromal/stem cells (MSCs). In this study, we investigated the effects of rapamycin on the proangiogenic ability of senescent human umbilical cord MSCs (UCMSCs). An in vitro replicative senescent cell model was established in cultured UCMSCs. We found that late passage (P25 or later) UCMSCs (LP-UCMSCs) exhibited impaired proangiogenic abilities. Treatment of P25 UCMSCs with rapamycin (900 nM) reversed the senescent phenotype and notably enhanced the proangiogenic activity of senescent UCMSCs. In a nude mouse model of hindlimb ischemia, intramuscular injection of rapamycin-treated P25 UCMSCs into the ischemic limb significantly promoted neovascularization and ischemic limb salvage. We further analyzed the changes in the expression of angiogenesis-associated genes in rapamycin-primed MSCs and found higher expression of several genes related to angiogenesis, such as VEGFR2 and CTGF/CCN2, in primed cells than in unprimed MSCs. Taken together, our data demonstrate that rapamycin is a potential drug to restore the proangiogenic activity of senescent MSCs, which is of importance in treating ischemic diseases and tissue engineering.
Similar content being viewed by others
Introduction
Mesenchymal stromal/stem cells (MSCs) were discovered in the bone marrow (BM) in 1966 [1] and have been shown to be broadly distributed throughout the body, including (but not limited to) dental pulp [2], umbilical cord blood [3], umbilical cord tissue, adipose tissue [4], the heart, the gut, the lung, the liver, the placenta, amniotic fluid [5] and periodontal ligaments. As MSCs have the potential to differentiate into bone, fat, cartilage, endodermic, and neuroectodermic cells and play an integral role in physiological processes, they have become a promising tool for cell-based therapy in tissue engineering and regenerative medicine [6]. A large number of studies have confirmed that MSCs promote angiogenesis and have been used to treat some diseases caused by impaired angiogenesis, such as peripheral artery disease (ischemia), myocardial infarction, and cerebral ischemia/stroke [7], and the safety, dose, and impact associated with MSC treatment in humans have been intensively studied in some published clinical trials [8]. Mechanistically, the beneficial proangiogenic effects of MSCs are predominantly caused by their broad repertoire of angiogenic factors, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), angiopoietin-1 (Ang-1), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6) and placental growth factor (PLGF) [9,10,11].
Senescence in MSCs impairs their therapeutic properties [12]. It has been reported that angiogenesis, wound healing, and proliferation were attenuated in aged MSCs compared to young MSCs [13]. Khan M et al. reported decreased tube formation by aged MSCs and the downregulation of insulin-like growth factor-1 (IGF-1), fibroblast growth factor (FGF-2), VEGF, and HGF compared to young MSCs. Mice transplanted with young MSCs exhibited significant improvements in left ventricular (LV) systolic and diastolic function, as demonstrated by dp/dt (max), dp/dt (min), and P (max). Reductions in the LV fibrotic areas were concomitant with neovascularization, as demonstrated by CD31 and smooth muscle actin (SMA) expression [14]. Senescent MSCs exhibit impaired proangiogenic ability that severely limits the efficacy of MSC transplantation to treat ischemic diseases.
Rapamycin (RAPA), an inhibitor of the mTOR (mammalian target of rapamycin) signaling pathway, is a macrolide antibiotic with potent immunosuppressive properties [15]. Recent studies have shown that rapamycin can slow certain aspects of cellular senescence. Inhibiting Akt/mTOR may mediate these effects by regulating the production of cytoplasmic ROS and the expression of the pluripotency genes Nanog and Oct-4 and reducing the accumulation of DNA damage during aging in MSCs [16]. Rapamycin can reverse the senescent phenotype of bone marrow-derived MSCs, which is associated with improved immunoregulation in the pathogenesis of systemic lupus erythematosus (SLE) [17]. Human MSCs cultured in the presence of rapamycin showed morphological characteristics of cells in early passages, retained their clonogenic ability, and showed high osteogenic potential [18], and rapamycin-primed MSCs could promote repair of the infarcted myocardium and effectively improve cardiac function [19]. However, it is unknown whether rapamycin can affect the proangiogenic ability of senescent MSCs. Here, we aimed to investigate the effects of rapamycin on the proangiogenic abilities of senescent UCMSCs.
Materials and methods
Isolation of UCMSCs and culture conditions
Human umbilical cords (UCs; n = 5) were obtained from consenting patients who delivered full-term (38–40 weeks) infants by cesarean section. The study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science, and Technology and followed the principles of the Declaration of Helsinki. The UCs were rinsed with phosphate-buffered saline (PBS; HyClone, Logan, UT, USA) to remove blood cells. Subsequently, the samples were cut into 1 cm segments, and the UC veins, arteries, and amnion were removed. The UCs were then minced into 1–3 mm3 pieces. The minced pieces were placed in a 55 cm2 culture dish and incubated in DMEM/F-12 (HyClone) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA). After 2 weeks, the UC tissue was removed, and the adherent cells were passaged with 0.25% trypsin (Gibco) for 1 min at 37 °C. The obtained cells were centrifuged at 250 ×g for 5 min and subcultured at a density of 4000 cells/cm2 in α-MEM (HyClone) supplemented with 5% human platelet lysate (HPL; Stemery Biotech, Fujian, China) and 100 U/mL penicillin/streptomycin (Gibco). The concentration of HPL was determined by CCK-8 analysis (Supplementary Fig. S1). Cell passaging was performed when the monolayer of adherent cells reached 70%–80% confluence. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
Culture of EA.hy926 cells
The human umbilical vein cell line EA.hy926 was purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in DMEM/high glucose (HyClone) supplemented with 10% FBS and 100 U/mL penicillin/streptomycin in a humidified incubator with 5% CO2/95% air at 37 °C. Cell passaging was performed when the monolayer of adherent cells reached 70%–80% confluence.
Flow cytometry analysis
UCMSCs and RAPA-UCMSCs were collected for phenotypic analysis. Phycoerythrin (PE)-labeled anti-CD29, CD44, CD73, CD90, CD105, CD106, CD34, CD45, and IgG isotype controls (all from BD Biosciences, San Diego, CA, USA) were incubated with UCMSCs and RAPA-UCMSCs for 30 min at 4 °C. After being washed in PBS three times, the cells were analyzed by flow cytometry (BD Biosciences).
For cell cycle analysis, UCMSCs and RAPA-UCMSCs were trypsinized, centrifuged, and washed three times in PBS. The cells were then resuspended and fixed in 70% ethanol at 4 °C overnight. The fixed cells were centrifuged at 500 ×g for 5 min, stained with 10 μg/mL PI, 100 μg/mL DNase-free RNase A, and 0.1% Triton X-100 and kept in the dark for 30 min. Cell suspensions were filtered and measured by FCM.
For cell apoptosis analysis, UCMSCs and RAPA-UCMSCs were trypsinized, centrifuged, and washed three times in PBS. The cells were then resuspended in 400 µL of binding buffer and 5 µL of Annexin V-FITC and incubated in the dark for 15 min at 4 °C. Then, 5 µL of PI reagent was added and mixed gently. The cells were incubated for 5 min at 4 °C in the dark. Cell suspensions were filtered and analyzed by FCM.
Flow cytometry data were analyzed by FlowJo 7.6 software (San Carlos, CA, USA).
Proliferation assay and conditioned medium preparation
Metformin (Sigma, St Louis, MO, USA) was dissolved in PBS at concentrations of 0, 0.05, 0.1, 0.5, and 1.0 mM, rapamycin (Sigma) was dissolved in dimethyl sulfoxide (DMSO) at concentrations of 0, 50, 100, 500, and 900 nM, the cytotoxicity of these compounds was examined compared to that in the control group. UCMSCs were seeded in 96-well plates at 1000 cells/well and cultured in the absence or presence of small molecules in standard UCMSC medium. At 1, 2, and 3 days posttreatment, the cell culture media was removed, and 10 μL of cell counting kit-8 (CCK-8, Dojindo, Rockville, MD, USA) solution and 100 μL of fresh cell culture media were added. The culture was maintained for another 2 h, the optical density (OD) at 450 nm was measured, and the final result was obtained by subtracting the background. Each sample was analyzed in quadruplicate. The optimal concentrations of small molecules were determined by choosing the concentration with the minimal effect on the UCMSC growth rate (Supplementary Fig. S1).
UCMSCs were cultured in growth media supplemented with rapamycin and metformin for 3 days and were cultured for another 24 h with fresh medium. Then, conditioned media (CM) without small molecules were collected. UCMSCs were cultured in deprivation of human platelet lysate (DHPL) conditions for 24 h, and then the CM was collected. UCMSCs cultured in growth media served as the control. The CM was centrifuged at 1800 rpm for 10 min to remove cell debris, filtered through 0.2 μm filters (Pall Corporation, Ann Arbor, MI, USA), and frozen at –80 °C.
To examine effects on proliferation, EA.hy926 cells were seeded at 1000 cells/well in a 96-well plate and cultured in DMEM supplemented with 10% FBS in the presence of CM. At 1, 2, and 3 days posttreatment, the cell culture media was removed, and 10 μL of cell counting kit-8 (CCK-8, Dojindo, Rockville, MD, USA) solution and 100 μL of fresh cell culture media were added. The culture was maintained for another 2 h, and OD at 450 nm was measured, and the final result was obtained by subtracting the background. Each sample was analyzed in quadruplicate.
Scratch wound healing assay
EA.hy926 cells were seeded at 2 × 105 cells/well in a six-well plate and cultured in DMEM supplemented with 10% FBS. After cell monolayer reached 100% confluence, a wound was created by manually scraping the monolayer with a P200 pipette tip. After the well was washed with PBS, EA.hy926 cells were cultured in DMEM for another 12 h in the presence of CM from UCMSCs, UCMSCs with DHPL, UCMSCs treated with metformin, and UCMSCs treated with rapamycin. Images were captured by a phase-contrast microscope (Olympus, Tokyo, Japan) at 0 h and 12 h after the scratch wound was made. The area of the wounded cell-free region was measured by ImageJ software (NIH, USA). Three independent experiments were performed, and each sample was analyzed in triplicate.
Tube formation assay
EA.hy926 cells were seeded at 5 × 104 cells/well in Matrigel (BD Biosciences, Bedford, MA, USA)-coated 24-well plates and cultured in DMEM in the presence of CM from UCMSCs and RAPA-UCMSCs. Three replicate wells were established for each group. After the cells were incubated for 12 h, photographs were taken by a microscope (Olympus, Melville, NY, USA), and the total length of the network was measured using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA) in five randomly selected fields in each well.
Senescence-associated β-galactosidase staining
The activity of senescence-associated β-galactosidase (β-gal) was measured using an SA-β-Gal staining kit (Beyotime, Shanghai, China). Briefly, UCMSCs (5 × 104 /mL) were grown in a 12-well plate and fixed for 15 min. Subsequently, the cells were washed with PBS and stained with SA-β-Gal staining solution at 37 °C for 12 h. Positive cells exhibited a blue color, and images were obtained with an inverted microscope (Olympus).
Enzyme-linked immunosorbent assay
The MCP-1 and IGFBP4 concentrations in CM were measured using enzyme-linked immunosorbent assay (ELISA; Abcam, Cambridge, UK) according to the manufacturer’s instructions. The results were obtained by measuring the absorbance at 450 nm. Each sample was measured in triplicate.
Real-time PCR
Total RNA was extracted from UCMSCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA quality and purity were examined using a Nucleic Acid/Protein Analyzer (Beckman Coulter, Brea, CA, USA); the A260/A280 ratio values were in the range of 1.8–2.0. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA per sample using a reverse transcription kit (Takara, Tokyo, Japan). The cDNA was amplified using Power SYBR Green PCR master mix (TIANGEN, Beijing, China) in an Applied Biosystems 7500 Real-Time PCR System, and each measurement was repeated in triplicate. GAPDH was used as an internal housekeeping gene, and the relative RNA expression levels were calculated using the ΔΔCt method. The specific primer sequences are shown in Supplementary Table S1.
Western blot analysis
UCMSCs and RAPA-UCMSCs were lysed in ice-cold RIPA buffer (Beyotime) for 30 min at 4 °C. Lysates (25 μg of protein) were resolved by 10% sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (Sigma) and transferred to nitrocellulose membranes (Merck Millipore, Billerica, MA, USA), which were hybridized with anti-PDGF (1:1000, Abcam, Cambridge, MA, USA), anti-VEGF (1:1000, Abcam), anti-VEGFR2 (1:1000, Affbiotech, Changzhou, Jiangsu, China), anti-CTGF (1:1000, Abcam), anti-ITGB3 (1:1000, Abcam), anti-EFNB2 (1:500, Affbiotech), anti-TGFBR1 (1:1000, Abcam), anti-TIMP-1 (1:500, Novus, Littleton, CO, USA), anti-HGF (1:1000, Abcam), anti-PGF (1:500, Abcam) or anti-GAPDH (1:10000, Abcam). The membranes were further incubated with HRP-coupled secondary antibodies (1:10000, ASPEN, Wuhan, China).
Preparation of the murine model of hindlimb ischemia
The animal studies were approved by the Ethics Committee of Huazhong University of Science and Technology, and all experiments were performed in accordance with relevant guidelines and regulations. A total of 6–7-week-old male BALB/c nude mice were anesthetized with 100 mg/kg sodium pentobarbital (Sigma). After an incision was made in the skin in the left inguinal region, the femoral artery was separated from the femoral vein and nerve. The proximal end of the femoral artery at the level of inguinal and distal to the saphenous artery was ligated and removed. Subsequently, the skin incision was closed with 5-0 silk sutures. The arteries on the right side were not ligated and served as controls.
Transplantation of RAPA-UCMSCs to treat limb ischemia
One day after arterial dissection, the mice were divided randomly into three experimental groups. The mice were randomly treated with injections of phosphate-buffered saline (PBS) (n = 8), UCMSCs (15 × 105 P25 UCMSCs in PBS, n = 8), and RAPA-UCMSCs (15 × 105 rapamycin-treated P25 UCMSCs in PBS, n = 8). The cells were washed with PBS several times before administration. PBS or cells were injected intramuscularly (im) into four sites of the gracilis muscle in the medial thigh of the ischemic limb. Ischemic damage and functional assessment of ischemic hindlimbs in each treatment group were assessed on Day 1, Day 7, Day 14, and Day 21. The physiological status of the ischemic limb was evaluated on Day 21 posttreatment. The outcome was rated at three levels: limb salvage (similar limb integrity and morphology as the normal control limb of the same animal), foot necrosis (death of foot tissue), or toe necrosis (death of toe tissue).
Histological staining
The ischemic gastrocnemius muscles in each group were harvested on Day 21 posttreatment, fixed in 4% paraformaldehyde overnight, and embedded in paraffin. Specimens were sliced into 8 mm-thick sections and stained with hematoxylin and eosin (H&E) to examine muscle degeneration and tissue inflammation. Masson’s trichrome collagen staining was performed to assess tissue fibrosis in ischemic regions. Images were taken under × 200 magnification.
Capillary density measurement
Immunohistochemistry was performed to assess capillary density. The sections were deparaffinized using xylene (dimethyl benzene) and heated in citrate buffer (pH 6.0) or 1 mmol/L EDTA (pH 8.0) to retrieve antigens. The slides were then blocked with 3% H2O2 and then 10% goat serum and incubated with the anti-α-SMA or an anti-CD31 primary antibodies for 1 h at room temperature or overnight at 4 °C. The sections were then washed with PBS and incubated with a secondary biotinylated anti-rabbit IgG for 15 min, followed by washing in PBS and incubation with an SABC kit (Boster, Wuhan, China). The signal was developed with a DAB chromogen kit (DAKO, Carpinteria, CA, USA), and the samples were counterstained with hematoxylin. Replacement of the primary antibody with PBS served as a negative control. The sections were observed under a microscope, five fields were randomly selected in each section, and CD31-positive cells and muscle fibers were counted in a blinded manner. The ratio of the number of CD31-positive cells to the number of myofibers was used as an index of capillary density.
Statistical analysis
The results are presented as the mean ± standard deviation. Two-tailed Student’s t test was conducted using GraphPad Prism 5 Software, and P values < 0.05 were considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). All results are representative of data generated from three independent experiments.
Results
Impaired proangiogenic abilities of UCMSCs induced by replicative senescence
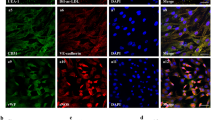
An in vitro replicative senescent cell model was established using long-term cultured UCMSCs. After passage 13 (P13), UCMSCs gradually became larger with flat and irregular shapes, which is the typical morphology of senescent cells (Fig. 1a). There were more β-galactosidase (β-gal)-stained cells in cultured UCMSCs after P25 than in earlier passages (Fig. 1b). The mRNA levels of p53, a senescence marker, were significantly increased in UCMSCs after long-term culture (P30 vs. P14, P < 0.001, Fig. 1c), with markedly increased protein levels of MCP-1 and IGFBP4, two senescence-associated secretory phenotype (SASP) markers (Fig. 1d).
a The morphology of UCMSCs at passages 14, 25, and 30 (P14, 25, and 30) was observed under an inverted microscope (×200; arrows indicate senescent cells). b β-gal staining (blue) of UCMSCs at P14, 25, and 30 was observed under an inverted microscope (×100; arrows indicate senescent cells). c RT–PCR analysis of the mRNA expression levels of p53; n = 3. d ELISA analysis of MCP-1 and IGFBP4 in UCMSCs at different passages (P14 and 30); n = 4. e The proliferation of EAhy926 cells cultured in the presence of the CM from EP-UCMSCs and LP-UCMSCs; n = 5. f RT–PCR analysis of the mRNA expression levels of ANG1, bFGF, PDGF, VEGF, and VEGFR2 in UCMSCs (P14 and 30); n = 3. g, h Representative images showing tube formation in response to CM from UCMSCs and quantitative analysis of the tube-like structures (three replicate wells for each group and five randomly selected views from each well were analyzed). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with P14; ###P < 0.001, ####P < 0.0001 compared with CTRL; $P < 0.05, $$$$P < 0.0001 compared with EP-UCMSCs.
In UCMSCs, it has not been reported whether angiogenesis could be impaired by replicative senescence. Thus, the endothelial cell line EAhy926 was cultured in the CM of UCMSCs. CCK-8 assays were performed to analyze the proliferation of cultured EAhy926 cells after 1, 2, and 3 days of CM treatment. The CM of late passage (P25 or later) UCMSCs (LP-UCMSCs) promoted less proliferation in EAhy926 cells than the CM of early passage (P14 or earlier) UCMSCs (EP-UCMSCs) (Fig. 1e). We also found that LP-UCMSCs expressed lower mRNA levels of the angiogenic growth factors ANG1, bFGF, PDGF, VEGF, and VEGFR2 than EP-UCMSCs (Fig. 1f). The in vitro Matrigel tube formation assay showed that the CM of LP-UCMSCs promoted fewer tube-like structures than that of EP-UCMSCs (Fig. 1g, h). These data confirmed the impaired angiogenic ability of UCMSCs induced by replicative senescence.
Rapamycin reverses the impaired proangiogenic abilities of long-term UCMSCs
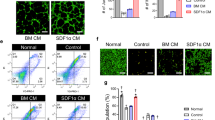
To determine whether rapamycin could affect the angiogenic ability of long-term UCMSCs, we chose P25 UCMSCs, which had typical characteristics of senescent cells, for experiments with the small molecule metformin (METF) and deprivation of human platelet lysate (DHPL) conditions as controls. The optimal concentrations of rapamycin and METF were determined by CCK-8 analysis of P25 UCMSCs (Supplementary Fig. S1). As shown in Fig. 2, morphology, β-gal staining, the mRNA levels of p53, and the protein levels of MCP-1 and IGFBP4 were compared among four groups of UCMSCs: P25 UCMSCs (CTRL), P25 UCMSCs with DHPL (DHPL), P25 UCMSCs treated with METF (METF), and P25 UCMSCs treated with rapamycin (RAPA). After rapamycin treatment, long-term UCMSCs became smaller and had a regular shape (Fig. 2a). Rapamycin reduced the number of β-gal-positive cells and significantly downregulated p53 mRNA expression and MCP-1 and IGFBP4 protein secretion in these cells (Fig. 2b, c, d). Flow cytometry (FCM) showed that both CTRL and RAPA cells were positive for CD29, CD44, CD73, CD90, and CD105 and negative for CD34 and CD45 (Fig. 2e), and there was increased expression of CD106 in RAPA cells (Fig. 2f). Compared to that in the CTRL group, the fraction of cells in G1/G0 was increased in the RAPA group (65.92% ± 2.76% vs. 58.64% ± 0.61%, P < 0.05), and there was a decrease in the fraction of cells in G2/S/M phase (RAPA vs. CTRL, 34.08% ± 2.76% vs. 41.36% ± 0.61%, P < 0.05, Fig. 2g) but no differences in apoptosis (Fig. 2h). These data demonstrate that rapamycin can successfully reverse the senescent phenotype of long-term UCMSCs.
a The morphology of UCMSCs in different groups was observed under an inverted microscope (×200; arrows indicate senescent cells). b β-gal staining (blue) of UCMSCs in the different groups was observed under an inverted microscope (×100; arrows indicate senescent cells). c RT–PCR analysis of the mRNA expression levels of p53 in different UCMSCs; n = 3. d ELISA analysis of MCP-1 and IGFBP4 in different UCMSCs; n = 4. e Immunophenotypic analysis of the surface markers CD29, CD44, CD73, CD90, CD105, CD34, and CD45 in the CTRL and RAPA groups by FCM. f Immunophenotypic analysis of the surface marker CD106 in the CTRL and RAPA groups by FCM. g Cell cycle characteristics in the CTRL and RAPA groups were examined by FCM. h Cell apoptosis in the CTRL and RAPA groups was analyzed by FCM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with the CTRL.
To investigate the in vitro angiogenic effects of long-term UCMSCs treated with rapamycin, EAhy926 cells were cultured in the presence of CM from phosphate-buffered saline (PBS)-washed UCMSCs after rapamycin, METF and DHPL treatment, and CM with human platelet lysate (HPL) was used as a control (CTRL). After 1, 2, and 3 days of CM treatment, the proliferation of EAhy926 cells in the RAPA group was more increased than that in the METF, DHPL, and CTRL groups (Fig. 3a). Both the mRNA and protein levels of PDGF, VEGF and VEGFR2 and VEGF secretion were significantly increased in the RAPA group (Fig. 3b, c, d, e). Then, scratch wound healing assays were performed to observe how the CM in the RAPA group affect the migration of EAhy926 cells. After 12 h of incubation, EAhy926 cells in the RAPA group had moved toward the cell-free area and closed the scratch wound by approximately 45.7%, which was much faster than that of cells in the CTRL group (16.9%, Fig. 3f, g). The in vitro Matrigel tube formation assays showed that after 12 h of incubation, more tube-like structures were observed in the RAPA group than in the CTRL group (Fig. 3h, i). These data confirmed that rapamycin could enhance the angiogenic activity of long-term UCMSCs in vitro.
a The proliferation of EAhy926 cells cultured in the presence of CM from UCMSCs in the different groups; n = 5. b Western blot analysis of the expression of PDGF, VEGF, and VEGFR2 in the CTRL and RAPA groups. c Statistical analysis of the Western blot results; n = 3. d RT–PCR analysis of the mRNA expression levels of PDGF, VEGF, and VEGFR2 in the different UCMSC groups; n = 3. e ELISA analysis of VEGF in the different UCMSCs; n = 3. f, g Representative images of a scratch wound healing assay in the different UCMSC groups and quantitative analysis of the percentage of wound healing; n = 5. h, i Representative images of tube formation in response to CM from CTRL and RAPA cells and quantitative analysis of the tube-like structures (three replicate wells for each group and five randomly selected views from each well were analyzed). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with CTRL; ##P < 0.01, ###P < 0.001 compared with UCMSCs.
Rapamycin-treated UCMSCs promote neovascularization and ischemic limb salvage in mice
To investigate whether RAPA-UCMSCs could enhance neovascularization in vivo, an ischemic hindlimb mouse model was used and randomly treated with injections of phosphate-buffered saline (PBS) (n = 8), UCMSCs (15 × 105 P25 UCMSCs in PBS, n = 8), and RAPA-UCMSCs (15 × 105 rapamycin-treated P25 UCMSCs in PBS, n = 8). Normal mice with normal hindlimbs that were not administered any injections (NATIVE) were used as controls. The injections were performed intramuscularly with the same volume into the medial thigh of the ischemic limb. Representative images on Days 1, 7, 14, and 21 after lower extremity artery ligation were used to examine the dynamic changes in the ischemic limb (Fig. 4a, b). On Day 1, the ischemic limbs lost the ability to walk normally, the temperature of the limbs decreased, and the skin appeared cyanotic in the three treatment groups. The degree of postoperative ischemic limb necrosis varied in the different treatment groups. On Day 7, redness and swelling of the ischemic limbs occurred with leg necrosis, foot necrosis, and toe necrosis in all groups. On Day 14, acute inflammation in the ischemic limbs was reduced, and most mice suffered auto amputation with leg necrosis (4 mice), foot necrosis (3 mice) and toe necrosis (1 mouse) in the PBS group; leg necrosis (0 mice), foot necrosis (5 mice) and toe necrosis (3 mice) in the MSC group; and leg necrosis (1 mouse), foot necrosis (2 mice), toe necrosis (3 mice) and limb salvage (2 mice) in the RAPA-MSC group. Statistical analysis of ischemic limb outcomes on Day 21 showed that in the PBS group, mice underwent foot necrosis (50%), toe necrosis (33.3%), or severe leg necrosis (16.7%) without limb salvage (0%). In the UCMSC group, the mice ultimately underwent foot necrosis (50%) or toe necrosis (50%). In sharp contrast, limb salvage (50%), leg necrosis (12.5%), foot necrosis (12.5%), or only mild toe necrosis (25%) were observed in the RAPA-UCMSC group (Fig. 4c). The average weight of mice in the RAPA-UCMSC group was higher than that in the PBS and UCMSC groups during the observation period (Fig. 4d).
a Representative images of ischemic limbs in each treatment group on Days 1, 7, 14, and 21; n = 8. b The enlarged image in the circled section in Fig. 4a. c Statistical analysis of ischemic limb outcomes in mice in each treatment group; n = 8. d The average weights of mice in each treatment group on Days 1, 7, 14, and 21; n = 8. e HE staining of muscle tissue in each treatment group (×200). f Masson staining of muscle tissue in each treatment group (×200). g α-SMA staining of muscle tissue in each treatment group (×200). h CD31 staining of muscle tissue on Days 7 and 14 (×200). i, j Quantitative analysis of CD31 staining in muscle tissues on Days 7 and 14 (three images of intact tissues were taken in each group, and five randomly selected views from each group were analyzed.) Image-Pro Plus 6.0 software was used to calculate the number of blood vessels and the tissue area, and the capillary density was determined. The data are shown as the mean ± SEM; n = 8 mice in each group. ***P < 0.001 compared with NATIVE; ###P < 0.001 compared with PBS; $$P < 0.01 compared with UCMSCs.
To examine histological changes in the ischemic regions, hematoxylin-eosin (H&E) staining, Masson staining, α-SMA staining, and CD31 staining were used to analyze the morphology, inflammation, fibrosis, smooth muscle hyperplasia, and neovascularization of muscle tissues, respectively. In the RAPA-UCMSC group, the histological pattern was similar to that in the NATIVE group, with mild muscle degeneration and inflammation but significantly more smooth muscle hyperplasia in the ischemic region than in the other two groups (Fig. 4e–g). Furthermore, to verify the angiogenic effects of transplanted cells, CD31+ vessel-like structures (number/mm2) were compared among the groups on Day 7 and Day 14 (Fig. 4h–j). On Day 7, the number of CD31+ vessels was much higher in the RAPA-UCMSC group than in the PBS and UCMSC groups (Fig. 4i). On Day 14, there were no significant differences in CD31+ vessel numbers between the RAPA-UCMSC group and the NATIVE group, and there was no acute inflammation in the ischemic limbs, suggesting recovery in the ischemic limbs. However, the number of CD31+ vessels in the PBS and UCMSC groups was much higher than that in the RAPA-UCMSC group (Fig. 4j), and there was a prolonged acute inflammatory phase in the ischemic limbs, suggesting extended healing time in these mice. These data demonstrated that rapamycin could enhance the activity of senescent UCMSCs to improve angiogenesis and ischemic limb salvage in vivo.
Rapamycin-induced changes in the expression of angiogenic genes in senescent UCMSCs
The expression of 84 genes related to angiogenesis in the UCMSC group and RAPA-UCMSC group was examined by a Human Angiogenesis RT2 ProfilerTM PCR Array (QIAGEN, Germany). When P < 0.05 was met, compared with that in the UCMSC group, 12 genes were upregulated more than 1.5-fold in the RAPA-UCMSC group, including KDR (VEGFR2), CTGF, EFNB2, ITGB3, TGFBR1, and TIMP1. Seventeen genes were downregulated more than 1.5-fold, including CCL11, CCL2 (MCP-1), CXCL1, PF4 (CXCL4), CXCL6, CXCL8, CXCL10, HGF, PGF, and PTGS1 (COX1) (Fig. 5a). GO function analysis and pathway analysis of the differentially expressed genes showed that the differences in molecular function were enriched in protein binding, and the cytokine–cytokine receptor interaction was significantly changed by RAPA treatment (Fig. 5b). Western blot analysis (Fig. 5c, d) based on the PCR microarray data revealed that the expression of the angiogenesis-related factors CTGF, ITGB3, EFNB2, and TIMP-1 was significantly increased in RAPA-UCMSCs. These data indicated that rapamycin could enhance the angiogenic activity of senescent UCMSCs by upregulating the expression of angiogenesis-related genes.
a The expression of angiogenesis-related genes in UCMSCs and RAPA-UCMSCs (n = 3). b Pathway analysis of the differential genes that were screened by specific criteria (fold change ≥ 1.5, P < 0.05). c Western blot analysis of the protein expression levels of CTGF, ITGB3, EFNB2, TGFBR1, TIMP-1, HGF, and PGF in UCMSCs and RAPA-UCMSCs. d Statistical analysis of the Western blot results (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with UCMSCs.
Discussion
Angiogenesis and neovascularization play major roles in wound healing and tissue repair and regeneration [20]. Insufficient vascularization in many diseases, such as critical limb ischemia, diabetic gangrene, myocardial infarction, and ischemic cerebrovascular disease, is often associated with poor clinical outcomes [21, 22]. Currently, conventional reperfusion treatments, including pharmaceutical (e.g., recombinant tissue plasminogen activator or streptokinase) or surgical approaches (e.g., stent placement or bypass grafting), are typically used to counteract these diseases [23, 24]. In addition, treatment attempts to promote angiogenesis and vasculogenesis use angiogenic growth factors (e.g., recombinant protein or gene therapy) [25]. MSCs are capable of secreting bioactive factors (especially VEGF) and differentiating into endothelial cells to facilitate angiogenesis, which can help to promote tissue regeneration and functional recovery in injured tissues [6, 7, 26], and tissue engineering based on MSC transplantation provides a promising therapeutic approach to accelerate angiogenesis [25]. However, senescence is one of the major challenges in the use of MSCs in regenerative medicine [12]. The decreased angiogenic capacity of senescent MSCs caused by aging or mass expansion in vitro severely limits their therapeutic applications in the treatment of ischemic diseases. To our knowledge, our study is the first to show that rapamycin can enhance the angiogenic activities of senescent UCMSCs in vitro, and presenescent MSCs that were pretreated with rapamycin could significantly promote angiogenesis and neovascularization, reduce the disability rate and shorten the recovery time of ischemic limbs in mice.
At present, relevant studies have been carried out to improve the angiogenic potency of MSCs by inducing lentivirus-mediated angiogenic genes [27,28,29] or regulating miRNAs associated with angiogenesis [30, 31]. However, many disappointing results have been produced in clinical trials. These vectors induced adverse effects, including toxicities and immune and oncogenicity, resulting in the termination of the trials [32]. In our study, we first used rapamycin, an emerging senolytic agent, to rejuvenate the angiogenic effects of senescent MSCs on wound healing. Compared with gene transfection, this approach is simple and efficient and has great application value for high-quality MSCs.
In this study, human platelet lysate (HPL) rather fetal bovine serum (FBS) was used to culture UCMSCs and simulate the in vitro amplification processes before the clinical application of MSCs. The angiogenic ability of expanded MSCs was weakened, and the SASP of MSCs decreased significantly after rapamycin treatment, which was consistent with the findings of previous reports. A recent study showed that specific subtypes of human MSCs, such as VCAM-1+ (vascular cell adhesion molecule 1, also known as CD106) cells, possess favorable angiogenic paracrine activity compared with other subtypes [33], and VCAM-1 expression was downregulated in senescent MSCs because of the reduced synthesis of hyaluronan [34]. Thus, we also found that CD106 expression in RAPA-UCMSCs was much higher than that in senescent UCMSCs. The most likely reason for the increase in CD106 on RAPA-MSCs is that rapamycin reverses senescent phenotypes and enhances the synthesis of hyaluronic acid in long-term UCMSCs, but related work is needed to verify this hypothesis. Furthermore, we used a Human Angiogenesis RT2 ProfilerTM PCR Array to examine the effect of rapamycin on the expression of angiogenesis-related genes in senescent UCMSCs and found that the expression levels of VEGFR2 (KDR), CTGF/CCN2, EFNB2, ITGB3, and TGFBR1 were upregulated in rapamycin-primed senescent MSCs.
Recent studies using cell labeling and single-cell technology supported the paracrine effects of MSC-mediated angiogenesis. The paracrine factors secreted by MSCs, including a core of angiogenic cytokines (i.e., VEGF, HGF, IL-8, TGFβ) and extracellular vesicles, might be the major contributors to this process [7]. Our previous studies identified extracellular vesicles (EVs) as key factors in the senescence-associated secretory phenotype of UCMSCs and demonstrated that their integrated characteristics can dynamically reflect the senescence state of parental UCMSCs [35]. We also found that coating DBM scaffolds with UCMSC-derived EVs could promote bone regeneration by accelerating vascularization [36]. Thus, EVs secreted by RAPA-UCMSCs may possess enhanced angiogenic abilities compared to those secreted by senescent UCMSCs. There is still much work to be done to verify this hypothesis.
There are some limitations to this study. It is unclear whether rapamycin can regulate the differentiation and migration of senescent MSCs into ischemic tissues, and further studies are necessary. Additionally, more preclinical pharmacological and toxicological studies of rapamycin-primed senescent MSCs are needed. Overall, our findings support the therapeutic potential of rapamycin-pretreated UCMSCs in ischemic disease and functional regeneration of damaged or diseased tissues.
References
Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966;16:381–90.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95.
Nadri S, Soleimani M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy. 2007;9:729–37.
Zhang ZY, Teoh SH, Hui JH, Fisk NM, Choolani M, Chan JK. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33:2656–72.
Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181–96.
Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–206.
Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–70.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59.
Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85.
Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16:445–53.
Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int. 2012;36:747–53.
Khan M, Mohsin S, Khan SN, Riazuddin S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. 2011;15:1515–27.
Kahan BD. Sirolimus: a new agent for clinical renal transplantation. Transpl Proc. 1997;29:48–50.
Gharibi B, Farzadi S, Ghuman M, Hughes FJ. Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells. 2014;32:2256–66.
Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging. 2016;8:1102–14.
Wang R, Yu Z, Sunchu B, Shoaf J, Dang I, Zhao S, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16:564–74.
Li ZH, Wang YL, Wang HJ, Wu JH, Tan YZ. Rapamycin-preactivated autophagy enhances survival and differentiation of mesenchymal stem cells after transplantation into infarcted myocardium. Stem Cell Rev Rep. 2020;16:344–56.
Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115–70.
Gupta AS. Nanomedicine approaches in vascular disease: a review. Nanomedicine. 2011;7:763–79.
Imori Y, Akasaka T, Ochiai T, Oyama K, Tobita K, Shishido K, et al. Co-existence of carotid artery disease, renal artery stenosis, and lower extremity peripheral arterial disease in patients with coronary artery disease. Am J Cardiol. 2014;113:30–35.
Rentrop KP, Feit F. Reperfusion therapy for acute myocardial infarction: Concepts and controversies from inception to acceptance. Am Heart J. 2015;170:971–80.
Lobo R, Kiernan TJ, Jaff MR. Medical therapy for critical limb ischemia and the diabetic foot: an update. J Cardiovasc Surg. 2013;54:671–8.
Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–91.
Yong KW, Choi JR, Mohammadi M, Mitha AP, Sanati-Nezhad A, Sen A, et al. Mesenchymal stem cell therapy for ischemic tissues. Stem Cells Int. 2018;2018:8179075.
Tang H, Xiang Y, Jiang X, Ke Y, Xiao Z, Guo Y, et al. Dual expression of hTERT and VEGF prolongs life span and enhances angiogenic ability of aged BMSCs. Biochem Biophys Res Commun. 2013;440:502–8.
Liang X, Ding Y, Lin F, Zhang Y, Zhou X, Meng Q, et al. Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 2019;33:4559–70.
Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, et al. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014;33:1083–92.
Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y, et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell. 2020;19:e13128.
Dong J, Zhang Z, Huang H, Mo P, Cheng C, Liu J, et al. miR-10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Res Ther. 2018;9:151.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le D, Wulffraat N, Mclntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6.
Du W, Li X, Chi Y, Ma F, Li Z, Yang S, et al. VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity. Stem Cell Res Ther. 2016;7:49.
Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK, Min JK, et al. Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells. Biochem Biophys Res Commun. 2011;404:463–9.
Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, et al. Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics. 2017;7:2673–89.
Xie H, Wang Z, Zhang L, Lei Q, Zhao A, Wang H, et al. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep. 2017;7:45622.
Acknowledgements
We thank Prof. Shun-chang Zhou, Tongji Medical College, Huazhong University of Science and Technology, for guiding the animal experimental procedures. This study was supported by the National Key Research and Development Program of China (No. 2021YFA1101500) and by the National Natural Science Foundation of China (Nos. 92049119 and 81974009 to Qiu-bai Li and 81974221 to Zhi-chao Chen).
Author information
Authors and Affiliations
Contributions
ZCC and QBL designed the study. YLC performed the experiments. YLC and WLC analyzed and interpreted the data. QL, FG, WXR, LC, HXW, and TC contributed scientific advice. YLC and QBL prepared the manuscript, and all authors approved and supported the decision to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
All authors certify that they have participated sufficiently in this work to take public responsibility for the content. The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Supplementary information
Rights and permissions
About this article
Cite this article
Cao, Yl., Chen, Wl., Lei, Q. et al. The transplantation of rapamycin-treated senescent human mesenchymal stem cells with enhanced proangiogenic activity promotes neovascularization and ischemic limb salvage in mice. Acta Pharmacol Sin 43, 2885–2894 (2022). https://doi.org/10.1038/s41401-022-00896-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00896-5
Keywords
This article is cited by
-
Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting
Cellular & Molecular Biology Letters (2022)