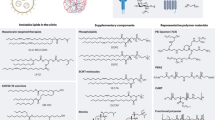
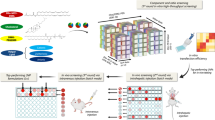
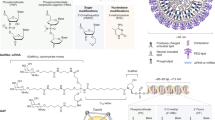
Abstract
Recent mechanistic studies have attempted to deepen our understanding of the process by which liposome-mediated delivery of genetic material occurs. Understanding the interactions between lipid nanoparticles and cells is still largely elusive. Liposome-mediated delivery of genetic material faces systemic obstacles alongside entry into the cell, endosomal escape, lysosomal degradation and nuclear uptake. Rational design approaches for targeted delivery have been developed to reduce off-target effects and enhance transfection. These strategies, which have included the modification of lipid nanoparticles with target-specific ligands to enhance intracellular uptake, have shown significant promise at the proof-of-concept stage. Control of physical and chemical specifications of liposome composition, which includes lipid-to-DNA charge, size, presence of ester bonds, chain length and nature of ligand complexation, is integral to the performance of targeted liposomes as genetic delivery agents. Clinical advances are expected to rely on such systems in the therapeutic application of liposome nanoparticle-based gene therapy. Here, we discuss the latest breakthroughs in the development of targeted liposome-based agents for the delivery of genetic material, paying particular attention to new ligand and cationic lipid design as well as recent in vivo advances.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Yang N . Nonviral gene delivery system. Int J Pharm Investig 2012; 2: 97–98.
Zylberberg C, Matosevic S . Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv 2016; 23: 3319–3329.
Šimčíková M, Prather KLJ, Duarte Prazeres MF, Monteiro GA . Towards effective non-viral gene delivery vector. Biotechnol Genet Eng Rev 2015; 31: 82–107.
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG . Non-viral vectors for gene-based therapy. Nat Rev Genet 2014; 15: 541–555.
Balasz DA, Godbey WT . Liposomes for use in gene delivery. J Drug Deliv 2011; 2011: Article ID 326497, 12 pages.
Liechty WB, Peppas NA . Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm 2012; 80: 241–246.
Channarong S, Chaicumpa W, Sinchaipanid N, Mitrevej A . Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: the improvement of peyer’s patch targeting using a polyplex-loaded liposomes. AAPS PharmSciTech 2011; 12: 192–200.
Rajala A, Wang Y, Zhu Y, Ranjo-Bishop M, Ma J-X, Mao C et al. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett 2014; 14: 5257–5263.
Alton EW, Stern MR, Farley R, Jaffe A, Chadwick SL, Phillips J et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet 1999; 353: 947–954.
Rädler JO, Koltover I, Salditt T, Safinya CR . Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 1997; 275: 810–814.
Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB et al. Nonbilayer phase of lipoplex—membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther 2005; 11: 801–810.
Ma B, Zhang S, Jiang H, Zhao B, Lv H . Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J Control Release 2007; 123: 184–194.
Ewert KK, Zidovska A, Ahmad A, Bouxsein NF, Evans HM, McAllister CS et al. Cationic lipid–nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNA. Top Curr Chem 2010; 296: 191–226.
Leventis L, Silvius JR . Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim Biophys Acta 1990; 1023: 124–132.
Byk G, Dubertret C, Escriou V, Frederic M, Jaslin G, Rangara R et al. Synthesis, activity, and structure-activity relationship studies of novel cationic lipids for DNA transfer. J Med Chem 1998; 41: 229–235.
Meka RR, Godeshala S, Marepally S, Thorat K, Rachamalla HKR, Dhayani A et al. Asymmetric cationic lipids based non-viral vectors for an efficient nucleic acid delivery. RSC Adv 2016; 6: 77841–77848.
Du Z, Munye MM, Tagalakis AD, Manunta MD, Hart SL . The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci Rep 2014; 4: 7107, 1–6.
Mochizuki S, Kanegae N, Nishina K, Kamikawa Y, Koiwai K, Masunaga H et al. The role of the helper lipid dioleoylphosphatidylethanolamine (DOPE) for DNA transfection cooperating with a cationic lipid bearing ethylenediamine. Biochim Biophys Acta 2013; 1828: 412–418.
Miller AD . The problem with cationic liposome/micelle-based non-viral vector systems for gene therapy. Curr Med Chem 2003; 10: 1195–1211.
Chan C-L, Ewert KK, Majzoub RN, Hwu YK, Liang KS, Leal C et al. Optimizing cationic and neutral lipids for efficient gene delivery at high serum content. J Gene Med 2014; 16: 84–96.
Prata CAH, Li Y, Luo D, McIntosh TJ, Barthelemy P, Grinstaff MW . A new helper phospholipid for gene delivery. Chem Commun 2008: (13): 1566–1568.
Silva JP, Oliveira AC, Casal MP, Gomes AC, Coutinho PJ, Coutinho OP et al. DODAB:monoolein-based lipoplexes as non-viral vectors for transfection of mammalian cells. Biochim Biophys Acta 2011; 1808: 2440–2449.
Barbeau J, Belmadi N, Montier T, Le Gall, Dalençon S, Lemiègre L et al. Synthesis of a novel archaeal tetraether-type lipid containing a diorthoester group as a helper lipid for gene delivery. Tetrahedron Lett 2016; 57: 2976–2980.
Brard M, Lainé C, Réthoré G, Laurent I, Neveu C, Lemiègre L et al. Synthesis of archaeal bipolar lipid analogues: a way to versatile drug/gene delivery systems. J Org Chem 2007; 72: 8267–8279.
Ghosh YK, Visweswariah SS, Bhattacharya S . Advantage of the ether linkage between the positive charge and the cholesteryl skeleton in cholesterol-based amphiphiles as vectors for gene delivery. Bioconjug Chem 2002; 13: 378–384.
Gopal V, Xavier J, Kamal MZ, Govindarajan S, Takafuji M, Shuta Soga S et al. Synthesis and transfection efficiency of cationic oligopeptide lipids: role of linker. Bioconjug Chem 2011; 22: 2244–2254.
Ivanova EA, Maslov MA, Kabilova TO, Puchkov PA, Alekseeva AS, Boldyrev IA et al. Structure-transfection activity relationships in a series of novel cationic lipids with heterocyclic head-groups. Org Biomol Chem 2013; 11: 7164–7178.
Zhi D, Zhang S, Qureshi F, Zhao Y, Cui S, Wang B et al. Synthesis and biological activity of carbamate-linked cationic lipids for gene delivery in vitro. Bioorg Med Chem Lett 2012; 22: 3837–3841.
Shi J, Yu S, Zhu J, Zhi D, Zhao Y, Cui S et al. Carbamate-linked cationic lipids with different hydrocarbon chains for gene delivery. Colloids Surf B Biointerfaces 2016; 141: 417–422.
Srujan M, Chandrashekhar V, Reddy RC, Prabhakar R, Sreedhar B, Chaudhuri A . The influence of the structural orientation of amide linkers on the serum compatibility and lung transfection properties of cationic amphiphiles. Biomaterials 2011; 32: 5231–5240.
Zhi D, Zhang S, Cui S, Zhao Y, Wang Y, Zhao D . The headgroup evolution of cationic lipids for gene delivery. Bioconjug Chem 2013; 24: 487–519.
Bajaj A, Mishra SK, Kondaiah P, Bhattacharya S . Effect of the headgroup variation on the gene transfer properties of cholesterol based cationic lipids possessing ether linkage. Biochim Biophys Acta 2008; 1778: 1222–1236.
Spelios M, Nedd S, Matsunaga N, Savva M . Effect of spacer attachment sites and pH-sensitive headgroup expansion on cationic lipid-mediated gene delivery of three novel myristoyl derivatives. Biophys Chem 2007; 129: 137–147.
Safinya CR, Ewert KK, Majzoub RN, Leal C . Cationic liposome–nucleic acid complexes for gene delivery and gene silencing. N J Chem 2014; 38: 5164–5172.
Berchel M, Le Gall T, Haelters JP, Lehn P, Montier T, Jaffrès PA . Cationic lipophosphoramidates containing a hydroxylated polar headgroup for improving gene delivery. Mol Pharm 2015; 12: 1902–1910.
Ewert K, Ahmad A, Evans HM, Schmidt HW, Safinya CR . Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J Med Chem 2002; 45: 5023–5029.
Shirazi RS, Ewert KK, Leal C, Majzoub RN, Bouxsein NF, Safinya CR . Synthesis and characterization of degradable multivalent cationic lipids with disulfide-bond spacers for gene delivery. Biochim Biophys Acta 2011; 1808: 2156–2166.
Matosevic S, Paegel BM . Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line. J Am Chem Soc 2011; 133: 2798–2800.
Belliveau NM, Huft J, Lin PJ, Chen S, Leung AK, Leaver TJ et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol Ther Nucleic Acids 2012; 1: e37.
Martin B, Sainlos M, Aissaoui A, Oudrhiri N, Hauchecorne M, Vigneron JP et al. The design of cationic lipids for gene delivery. Curr Pharm Des 2005; 11: 375–394.
Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 2010; 28: 172–176.
Guo X, Gagne L, Chen H, Szoka FC . Novel ortho ester-based, pH-sensitive cationic lipid for gene delivery in vitro and in vivo. J Liposome Res 2014; 24: 90–98.
Malamas AS, Gujrati M, Kummitha CM, Xu R, Lu Z-R . Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J Control Release 2013; 171: 296–307.
Kuramoto Y, Nishikawa M, Hyoudou K, Yamashita F, Hashida M . Inhibition of peritoneal dissemination of tumor cells by single dosing of phosphodiester CpG oligonucleotide/cationic liposome complex. J Control Release 2006; 115: 226–233.
Qiu Y, Guo L, Zhang S, Xu B, Gao Y, Hu Y et al. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv 2016; 23: 2391–2398.
Hatakeyama H, Akita H, Harashima H . The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull 2013; 36: 892–899.
Chan C-L, Majzoub RN, Shirazi RS, Ewert KK, Chen YJ, Liang KS et al. Endosomal escape and transfection efficiency of PEGylated cationic lipid–DNA complexes prepared with an acid-labile PEG-Lipid. Biomaterials 2012; 33: 4928–4935.
Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H . Dual ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release 2011; 153: 141–148.
Yingyuad P, Mével M, Prata C, Kontogiorgis C, Thanou M, Miller AD . Enzyme-triggered PEGylated siRNA-nanoparticles for controlled release of siRNA. J RNAi Gene Silencing 2014; 10: 490–499.
Franzen S . A comparison of peptide and folate receptor targeting of cancer cells: from single agent to nanoparticle. Expert Opin Drug Deliv 2011; 8: 281–298.
Kim BK, Hwang GB, Seu YB, Choi JS, Jin KS, Doh KO . DOTAP/DOPE ratio and cell type determine transfection efficiency with DOTAP-liposomes. Biochim Biophys Acta 2015; 1848 (10 Pt A): 1996–2001.
Park J, Singha K, Son S, Kim J, Namgung R, Yun CO et al. A review of RGD-functionalized nonviral gene delivery vectors for cancer therapy. Cancer Gene Ther 2012; 19: 741–748.
Amin M, Mansourian M, Koning GA, Badiee A, Jaafari MR, ten Hagen TL . Development of a novel cyclic RGD peptide for multiple targeting approaches of liposomes to tumor region. J Control Release 2015; 220 (Part A): 308–315.
Yonenaga N, Kenjo E, Asai T, Tsuruta A, Shimizu K, Dewa T et al. RGD-based active targeting of novel polycation liposomes bearing siRNA for cancer treatment. J Control Release 2012; 160: 177–181.
Sakurai Y, Hatakeyama H, Sato Y, Hyodo M, Akita H, Ohga N et al. RNAi-mediated gene knockdown and anti-angiogenic therapy of RCCs using a cyclic RGD-modified liposomal-siRNA system. J Control Release 2014; 73: 110–118.
LaManna CM, Lusic H, Camplo M, McIntosh TJ, Barthélémy P, Grinstaff MW . Charge-reversal lipids, peptide-based lipids, and nucleoside-based lipids for gene delivery. Acc Chem Res 2012; 45: 1026–1038.
Muñoz-Úbeda M, Misra SK, Barrán-Berdón AL, Datta S, Aicart-Ramos C, Castro-Hartmann P et al. How does the spacer length of cationic gemini lipids influence the lipoplex formation with plasmid DNA? Physicochemical and biochemical characterizations and their relevance in gene therapy. Biomacromolecules 2012; 13: 3926–3937.
Zhao Y-N, Qureshi F, Zhang S-B, Cui SH, Wang B, Chen HY et al. Novel gemini cationic lipids with carbamate groups for gene delivery. J Mater Chem B Mater Biol Med 2014; 2: 2920–2928.
Misra SK, Kondaiah P, Santanu Bhattacharya S, Boturyn D, Dumy P . Co-liposomes comprising a lipidated multivalent RGD-peptide and a cationic gemini cholesterol induce selective gene transfection in αvβ3 and αvβ5 integrin receptor-rich cancer cells. J Mater Chem B 2014; 2: 5758–5767.
Nakase I, Akita H, Kogure K, Gräslund A, Langel U, Harashima H et al. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc Chem Res 2012; 45: 1132–1139.
Mishra A, Lai GH, Schmidt NW, Sun VZ, Rodriguez AR, Tong R et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc Natl Acad Sci USA 2011; 108: 16883–16888.
El-Sayed A, Futaki S, Harashima H . Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. 2009 AAPS J 11: 13–22.
Tunnemann G, Ter-Avetisyan G, Martin RM, Stockl M, Herrmann A, Cardoso MC . Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J Pept Sci 2008; 14: 469–476.
Shin MC, Zhang J, Min KA, Lee K, Byun Y, David AE et al. Cell-penetrating peptides: achievements and challenges in application for cancer treatment. J Biomed Mater Res A. 2014; 102: 575–587.
Moon IJ, Kang H, Seu YB, Chang BC, Song DK, Park JG . Marked transfection enhancement by the DPL (DNA/peptide/lipid) complex. Int J Mol Med 2007; 20: 429–437.
Lee SJ, Yoon SH, Doh KO . Enhancement of gene delivery using novel homodimeric Tat peptide formed by disulfide bond. J Microbiol Biotechnol 2011; 21: 802–807.
Saleh AF, Aojula H, Arthanari Y, Offerman S, Alkotaji SM, Pluen A . Improved Tat-mediated plasmid DNA transfer by fusion to LK15 peptide. J Control Release 2010; 143: 233–242.
Hirose H, Takeuchi T, Osakada H, Pujals S, Katayama S, Nakase I et al. Transient focal membrane deformation induced by arginine-rich peptides leads to their direct penetration into cells. Mol Ther 2012; 20: 984–993.
Palm-Apergi C, Lönn P, Dowdy SF . Do cell-penetrating peptides actually “Penetrate” cellular membranes? Mol Ther 2012; 20: 695–697.
Negishi Y, Omata D, Iijima H, Hamano N, Endo-Takahashi Y, Nomizu M et al. Preparation and characterization of laminin-derived peptide AG73- coated liposomes as a selective gene delivery tool. Biol Pharm Bull 2010; 33: 1766–1769.
Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B et al. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: biodistribution and transfection. J Control Release 2013; 167: 1–10.
Qin L, Wang C-Z, Fan H-J, Zhang C-J, Zhang H-W, M-H LV et al. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol Lett 2014; 8: 2000–2006.
Manjappa AS, Chaudhari KR, Venkataraju MP, Dantuluri P, Nanda B, Sidda C et al. Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J Control Release 2011; 150: 2–22.
Ansell SA, Harasym TO, Tardi PG, Buchkowsky SS, Bally MB, Cullis PR. Antibody conjugation methods for active targeting of liposomes. Methods in molecular medicine. In: Francis GE, Delgado CO (eds). Drug Targeting: Strategies, Principles, and Applications, vol. 25. Humana Press Inc.: Totowa, NJ, USA..
Ko YT, Hartner WC, Kale A, Torchilin VP . Gene delivery into ischemic myocardium by double-targeted lipoplexes with anti-myosin antibody and TAT peptide. Gene Therapy 2009; 16: 52–59.
Saeed M, van Brakel M, Zalba S, Schooten E, Rens JAP, Koning GA et al. Targeting melanoma with immunoliposomes coupled to anti-MAGE A1 TCR-like single-chain antibody. Int J Nanomed 2016; 11: 955–975.
Kim KS, Park YS, Hong HJ, Kim KP, Lee KH, Kim DE . Enhanced tumor-targeted gene delivery by immunolipoplexes conjugated with the humanized anti-TAG-72 Fab' fragments. Bull Korean Chem Soc 2012; 33: 651–656.
Yoon YI, Kwon Y-S, Cho H-S, Heo SH, Park KS, Park SG et al. Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics 2014; 4: 1133–1144.
Yue P, He L, Qiu S, Li Y, Liao YJ, Li XP et al. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol Cancer 2014; 13: 191.
Pardridge WM . Preparation of Trojan horse liposomes (THLs) for gene transfer across the blood-brain barrier. Cold Spring Harb Protoc 2010; 2010: pdb.prot5407.
Boado RJ, Pardridge WM . The Trojan Horse Liposome Technology for nonviral gene transfer across the blood-brain barrier. J Drug Deliv 2011; 2011: Article ID 296151, 12 pages.
Skjørringe T, Gjetting T, Jensen TG . A modified protocol for efficient DNA encapsulation into pegylated immunoliposomes (PILs). J Control Release 2009; 139: 140–145.
Ko YT, Bhattacharya R, Bickel U . Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J Control Release 2009; 133: 230–237.
Zang X, Ding H, Zhao X, Li X, Du Z, Hu H et al. Anti-EphA10 antibody-conjugated pH-sensitive liposomes for specific intracellular delivery of siRNA. Int J Nanomed 2016; 11: 3951–3967.
Ara MN, Matsuda T, Hyodo M, Sakurai Y, Hatakeyama H, Ohga N et al. An aptamer ligand based liposomal nanocarrier system that targets tumor endothelial cells. Biomaterials 2014; 35: 7110–7120.
Prakash JS, Rajamanickam K . Aptamers and their significant role in cancer therapy and diagnosis. Biomedicines 2015; 3: 248–269.
Esposito CL, Catuogno S, de Franciscis V . Aptamer-mediated selective delivery of short RNA therapeutics in cancer cells. J RNAi Gene Silencing 2014; 10: 500–506.
Wilner SE, Wengerter B, Maier K, de Lourdes Borba Magalhães M, Del Amo DS, Pai S et al. An RNA alternative to human transferrin: a new tool for targeting human cells. Mol Ther Nucleic Acids 2012; 1: e21.
Li L, Hou J, Liu X, Guo Y, Wu Y, Zhang L et al. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014; 35: 3840–3850.
Liu YJ, Dou XQ, Wang F, Zhang J, Wang XL, Xu GL et al. IL-4Rα aptamer-liposome-CpG oligodeoxynucleotides suppress tumour growth by targeting the tumour microenvironment. J Drug Target 2017; 25: 275–283.
He Z, Yu Y, Zhang Y, Yan Y, Zheng Y, He J et al. Gene delivery with active targeting to ovarian cancer cells mediated by folate receptor alpha. J Biomed Nanotechnol 2013; 9: 833–844.
Gorle S, Ariatti M, Singh M . Folate Receptor Targeted Gene Delivery Using Cationic Liposomes as Nonviral Vectors. 2nd International Conference on Geological and Environmental Sciences IPCBEE: Hong Kong, 2013.
Yang JP, Huang L . Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Therapy 1997; 4: 950–960.
Urbiola K, García L, Zalba S, Garrido MJ, Tros de Ilarduya C . Efficient serum-resistant lipopolyplexes targeted to the folate receptor. Eur J Pharm Biopharm 2013; 83: 358–363.
Cui SH, Zhi DF, Zhao YN, Chen HY, Meng Y, Zhang CM et al. Cationic lioposomes with folic acid as targeting ligand for gene delivery. Bioorg Med Chem Lett 2016; 26: 4025–4029.
Zhang Y, Chatterjee DK Liposomes, Dendrimers and other Polymeric Nanoparticles for Targeted Delivery of Anticancer Agents – A Comparative Study in Nanotechnologies for the Life Sciences. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany 2007..
Li F, Wang Z, Huang Y, Xu H, He L, Deng Y et al. Delivery of PUMA apoptosis gene using polyethyleneimine-SMCC-TAT/DNA nanoparticles: biophysical characterization and in vitro transfection into malignant melanoma cells. J Biomed Nanotechnol 2015; 11: 1776–1782.
Jin L, Zeng X, Liu M, Deng Y, He N . Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics 2014; 4: 240–255.
Li F, Jin L, He L, Deng Y, He N . Nanoparticles applied for therapy and diagnosis in common diseases. Sci Adv Mater 2015; 7: 2103–2122.
Kim Y-K, Zhang M, Zhang B-F, Wang F-Z, Cui P-F, Xie R-L et al. Hepatic targeting gene delivery using galactosylated ester-based polyspermine. J Nanosci Nanotechnol 2016; 16: 6955–6963.
Yasuma R, Cicatiello RV, Mizutani T, Tudisco L, Kim Y, Tarallo V et al. Intravenous immune globulin suppresses angiogenesis in mice and humans. Signal Transduct Target Ther 2016; 1: 15002.
Peng Y, Croce CM . The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016; 1: 15004.
Sharma P, Banerjee R, Narayan KP . Data for stable formulation of steroid hormone receptor targeted liposomes for cancer therapeutics. Data Brief 2016; 7: 428–431.
Yang T, Li B, Qi S, Liu Y, Gai Y, Ye P et al. Co-delivery of doxorubicin and Bmi1 siRNA by folate receptor targeted liposomes exhibits enhanced anti-tumor effects in vitro and in vivo. Theranostics 2014; 4: 1096–1111.
Zhang L, Li Z, Sun F, Xu Y, Du Z . Effect of inserted spacer in hepatic cell-penetrating multifunctional peptide component on the DNA intracellular delivery of quaternary complexes based on modular design. Int J Nanomed 2016; 11: 6283–6295.
Gorle S, Sewbalas A, Ariatti M, Singh M . Ligand-tagged cationic liposome facilitates efficient gene delivery to folate receptors. Curr Sci 2016; 111: 662–670.
Oh HR, Jo H-Y, Park JS, Kim D-E, Cho J-Y, Kim P-H et al. Galactosylated liposomes for targeted co-delivery of doxorubicin/vimentin siRNA to hepatocellular carcinoma. Nanomaterials 2016; 6: 141.
Zhang W, Peng F, Zhou T, Huang Y, Zhang L, Ye P et al. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. Int J Nanomed 2015; 10: 4825–4836.
Pappalardo JS, Langellotti CA, Di Giacomo S, Olivera V, Quattrocchi V, Zamorano PI et al. In vitro transfection of bone marrow-derived dendritic cells with TATp-liposomes. Int J Nanomed 2014; 9: 963–973.
Mendez N, Herrera V, Zhang L, Hedjran F, Feuer R, Blair SL et al. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials 2014; 35: 9554–9561.
Adil M, Belur L, Pearce TRC, Levine RM, Tisdale AW, Sorenson BS et al. PR_b functionalized stealth liposomes for targeted delivery to metastatic colon cancer. Biomater Sci 2013; 1: 393–401.
Li Y, Liu R, Shi Y, Zhang Z, Zhang X . Zwitterionic Poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics 2015; 5: 583–596.
Kono Y, Kawakami S, Higuchi Y, Maruyama K, Yamashita F, Hashida M . Tumour associated macrophages targeted transfection with NF-κB decoy/mannose-modified bubble lipoplexes inhibits tumour growth in tumour-bearing mice. J Drug Target 2014; 22: 439–449.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zylberberg, C., Gaskill, K., Pasley, S. et al. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther 24, 441–452 (2017). https://doi.org/10.1038/gt.2017.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.41
This article is cited by
-
Nanoparticle delivery for central nervous system diseases and its clinical application
Nano Research (2024)
-
Probing neural circuit mechanisms in Alzheimer’s disease using novel technologies
Molecular Psychiatry (2023)
-
Gene knockdown in HaCaT cells by small interfering RNAs entrapped in grapefruit-derived extracellular vesicles using a microfluidic device
Scientific Reports (2023)
-
Invasion by exogenous RNA: cellular defense strategies and implications for RNA inference
Marine Life Science & Technology (2023)
-
Recent advances in near-infrared-II hollow nanoplatforms for photothermal-based cancer treatment
Biomaterials Research (2022)