Abstract
Ultrasound-targeted microbubble destruction (UTMD) was used to direct the delivery of plasmid and transposase-based vectors encoding human factor IX (hFIX) to the livers of hemophilia B (FIX−/−) mice. The DNA vectors were incorporated into cationic lipid microbubbles, injected intravenously, and transfected into hepatocytes by acoustic cavitation of the bubbles as they transited the liver. Ultrasound parameters were identified that produced transfection of hepatocytes in vivo without substantial damage or bleeding in the livers of the FIX-deficient mice. These mice were treated with a conventional expression plasmid, or one containing a piggyBac transposon construct, and hFIX levels in the plasma and liver were evaluated at multiple time points after UTMD. We detected hFIX in the plasma by western blotting from mice treated with either plasmid during the 12 days after UTMD, and in the hepatocytes of treated livers by immunofluorescence. Reductions in clotting time and improvements in the percentage of FIX activity were observed for both plasmids, conventional (4.15±1.98%), and transposon based (2.70±.75%), 4 to 5 days after UTMD compared with untreated FIX (−/−) control mice (0.92±0.78%) (P=0.001 and P=0.012, respectively). Reduced clotting times persisted for both plasmids 12 days after treatment (reflecting percentage FIX activity of 3.12±1.56%, P=0.02 and 3.08±0.10%, P=0.001, respectively). Clotting times from an additional set of mice treated with pmGENIE3-hFIX were evaluated for long-term effects and demonstrated a persistent reduction in average clotting time 160 days after a single treatment. These data suggest that UTMD could be a minimally invasive, nonviral approach to enhance hepatic FIX expression in patients with hemophilia.
Similar content being viewed by others
Introduction
Hemophilia B is an X-linked blood coagulation disorder resulting from a deficiency in blood coagulation factor IX (FIX) that affects ∼1 in 25 000 males, a total of 3300 individuals in the United States.1, 2 Most affected individuals have a severe form of the disease and suffer from frequent and spontaneous bleeding episodes that can result in serious complications including debilitating arthropathy and intracerebral hemorrhage. Current treatment requires frequent infusions of FIX protein concentrates, up to several times per week. This is inconvenient and expensive and can lead to immune responses to FIX.3, 4 An alternative and more durable therapy could substantially improve the quality of life for affected individuals.
Hemophilia B is an attractive target for gene therapy because it results from a single gene mutation, there are animal models available, and restoration of FIX to as little as 1% of the normal levels can reduce bleeding episodes. Viral vectors have been used to mediate FIX gene transfer.5 Nathwani et al.4, 6 reported on a successful clinical gene therapy trial using an adenovirus-associated vector encoding human FIX (hFIX) in six hemophilia B patients, observing expression of hFIX ranging from 2 to 11% of the normal level. An update to this study including an additional four patients on the highest dose was recently reported that suggested long-term and substantial clinical benefit from genetic replacement therapy.6 Most recently, promoterless gene targeting in mice, mediated by adeno-associated virus, successfully delivered and integrated FIX to a site under the regulation of the albumin promoter, with subsequent robust expression.7 Although promising, specific limitations of viral-based gene transfer approaches include risk of liver toxicity from the viral vector, neutralizing antibodies that can limit transduction and duration of expression, the systemic nature of the therapy, and the potential for insertional mutagenesis.
Nonviral DNA vectors may be safer than viruses, with lower immunogenicity when delivering therapeutic transgenes such as FIX, but they do not achieve the transfection efficiency, duration, and gene expression levels obtained with viral vectors. To address these limitations, DNA transposons, integrases and minicircle vectors have been evaluated as transgene vectors to deliver FIX to the liver.8, 9, 10, 11, 12, 13, 14
In these studies, we evaluate ultrasound-targeted microbubble destruction (UTMD) for the hepatic delivery of liver-specific conventional and transposon hFIX vectors.15, 16 UTMD is minimally invasive and can efficiently evaluate a broad range of nonviral gene delivery vectors with highly specific tissue targeting. UTMD is performed at clinically tolerated ultrasound energy, with bubbles that are known to be safe, suggesting that it could ultimately be included in a combined approach, incorporating optimized delivery vectors for human therapy. Briefly, in UTMD, a vector encoding a gene of interest is added to the shell of cationic lipid microbubbles; these are then injected intravenously and the construct is deposited at the target organ by acoustic cavitation at a resonant frequency of the bubbles.16 In previous studies, UTMD has been used to deliver luciferase, green fluorescent protein and β-galactosidase reporter genes and potentially therapeutic genes, such as vascular endothelial growth factor, Hexokinase I, stem cell factor, stromal cell-derived factor-1α and Factor IX.9, 17, 18, 19, 20 Findings from these studies include short-term gene expression and higher transfection efficiency compared with using plasmid DNA alone; however, further enhancement of the technique is necessary to obtain therapeutic levels and duration of expression. Advantageously, UTMD can also be combined with additional advances in vector technology and gene delivery, such as tissue-specific promoters and transposon-based vectors, to improve the efficacy of gene transfer.21
Results
Confirmation of FIX deletion and phenotype
We obtained the FIX knockout mice from The Jackson Laboratory (Strain: B6.129P2-F9tm1Dws/J, Stock No. 004303, Bar Harbor, ME, USA) and confirmed loss of FIX in the knockout model at the DNA, RNA, and protein levels. We also confirmed that these mice had a hemophiliac phenotype by bleeding and clotting time assays (Supplementary Figure 1). The bleeding time assay demonstrated a two-thirds larger blood loss collected from mutant mice (12.5 μl) compared with wild-type mice (7.5 μl) over 5 min after a distal tail transection (Supplementary Figure 1). An activated partial thromboplastin time (APTT) assay was used to determine average clotting times. These were 48.1±3.4 s for wild-type (n=22) and 136.1±12.1 s for mice with the homozygous deletion (n=6; Table 1).
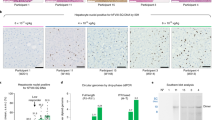
Livers were collected from wild-type and mutant mice to serve as histological controls, demonstrating the absence of FIX protein in mutant mice and the lack of crossreactivity between the antibody for human FIX and murine FIX (Figures 1a and b) compared with a human FIX UTMD-treated mutant mouse liver 1 day after treatment (× 40, Figure 1c). Hematoxylin and eosin (H&E) staining of liver showed small regions of hemorrhage and necrosis in the livers of hemophiliac mice before any treatment with UTMD (Figures 1d and e). Minimal tissue damage was observed by H&E staining in an additional control mutant mouse that received the liver-specific reporter only by UTMD (Figure 1f).
Histological discrimination of human FIX expression from mouse FIX and liver histology of wild-type and mutant control mice. (a) Untreated wild-type mouse left liver lobe stained with hFIX antibody, × 40. (b) Untreated FIX mutant mouse left liver lobe stained with hFIX antibody, × 40. (c) Human FIX UTMD-treated mutant mouse left liver lobe stained with hFIX antibody at 1 day after treatment, × 40. (d, e) Immunofluorescence and H&E-stained left liver lobes from untreated mice demonstrate the absence of hFIX expression and antibody crossreactivity to murine FIX (mFIX). (f) hFIX negative control histology. H&E stain of a mutant mouse liver 1 day after treatment with pZY53-luc and microbubbles (without hFIX) followed by UTMD.
UTMD-mediated delivery of hFIX to the livers of FIX (−/−) mice
We delivered hFIX to the livers of FIX (−/−) mice by UTMD using a liver-specific conventional expression construct, pZY53-hFIX, and a piggyBac transposon plasmid, pmGENIE3-hFIX. The plasmids were co-delivered with a liver-specific luciferase reporter, pZY53-luc, to confirm hepatic transfection by bioluminescence. Figure 2 demonstrates liver-specific reporter expression 2 days (Figure 2a) and 5 days (Figure 2b) after UTMD. Immunofluorescence and H&E staining were conducted at day 5 to evaluate hFIX transgene expression and localization as well as tissue damage from acoustic exposure or transgene delivery. Substantial hFIX transgene expression was detected in the left liver lobe by immunofluorescence, with more transfected cells located near hepatic blood vessels (Figures 2c and d) compared with primary antibody negative control (Figure 2d). This observation is consistent with our previous findings pertaining to reporter expression and localization in hepatocytes from mice transfected with a variety of luciferase vectors by UTMD.15 Importantly, H&E staining of the left liver lobe did not reveal any significant tissue damage 5 days after the UTMD treatment (Figures 2f–h). Reporter expression in a mutant mouse liver at 1 and 12 days after UTMD-mediated co-delivery with pmGENIE3-hFIX is shown in Figures 3a and b. Immunofluorescence revealed positive hFIX expression in hepatocytes in the left and medial liver lobes 1 day after treatment with pmGENIE3-hFIX (Figure 3c). H&E staining also showed an increase in tissue damage, most likely the result of regional hemorrhage and/or coagulative necrosis 1 day after UTMD delivery of pmGENIE3-hFIX (Figure 3d). Tissue damage appeared to be localized near regions of increased hFIX transgene expression. Tissue damage observed 1 day after UTMD treatment in the mutant mouse liver was greater than what was previously seen in wild-type mice (in which damage was virtually absent) and, interestingly, was greater than seen in a control mutant mouse receiving the reporter only by UTMD (Figure 1e). It appears that the mutant mouse model is more susceptible to the effects of UTMD-related acoustic exposure and/or hFIX plasmid delivery than wild-type mice.
Co-delivery of liver-specific hFIX and reporter vectors to the mutant mouse liver. (a, b) Expression from pZY53-luc (co-delivered with pZY53-hFIX) 2 (a) and 5 (b) days after UTMD. Luciferase expression (luc) is measured as the average radiance in units of photons per s per cm2 per steradian. (c–e) hFIX expression in the mutant mouse liver by immunofluorescence. Left liver lobe immunofluorescence from treated mutant mouse 5 days after UTMD-mediated delivery of pZY53-hFIX. Hepatocytes expressing the hFIX transgene appear green and were incubated with a goat anti-human FIX primary antibody and then stained with an Alexa Fluor 488 fluorescent-conjugated secondary antibody, and nuclei are stained blue with DAPI, × 40 magnification (c, d). White arrows indicate hFIX transgene expression surrounding hepatic blood vessels. Negative control (e), liver section stained only with the secondary antibody and DAPI. (f–h) H&E staining of mutant mouse liver 5 days after UTMD-mediated delivery of hFIX (pZY53-hFIX). Left liver lobe sections, scale=50 μm (f, g) and 100 μm (h).
Co-delivery of liver-specific hFIX and reporter vectors to the mutant mouse liver. (a, b) Expression from pZY53-luc (co-delivered with pmGENIE3-hFIX) at 1 (a) and 12 (b) days after UTMD. Luciferase expression (luc) is measured as the average radiance in units of photons per s per cm2 per steradian. (c) Immunofluorescence images of hFIX expression in the left and medial lobes (× 10–40) of the mutant mouse liver 1 day after treatment with pmGENIE3-hFIX. (d) H&E staining of mutant mouse liver 1 day after UTMD-mediated delivery of hFIX (pmGENIE3-hFIX). Top panel, left liver lobe sections (× 10–40), middle panel, medial liver lobe sections (× 10–40), and bottom panel, right liver lobe sections (× 10–40), scale=100 μm (× 10) and 50 μm (× 20–40).
hFIX expression in treated FIX (−/−) mouse plasma
We observed protein bands for hFIX (~55 kDa) and the hFIX heavy chain (~28 kDa) in the plasma from mutant mice treated with pmGENIE3-hFIX at 1, 4 or 5 and 12 days after UTMD (Figures 4a and b). Relative hFIX expression was statistically significant in pmGENIE3-hFIX-treated mice 1, 4 or 5 and 12 days after UTMD compared with untreated mutant mice (Figure 4b, P=0.04). The greatest expression by western blot was seen at the intermediate time point. The western blot from plasma samples collected from mice treated with the conventional pZY53-hFIX is shown in Figure 5. The strongest expression was again observed for samples collected 4 or 5 and 12 days after UTMD. The strongest expression levels (two of the day 4 to 5 samples and two of the day 12 samples) correspond to samples that also had the greatest reductions in clotting times (Figure 6). A statistically significant correlation between the relative hFIX expression and the clotting times was observed in plasma samples from mice treated with pZY53-hFIX (Figure 6b, P=0.035), but was not apparent for the pmGENIE3-hFIX treatments (Figure 6a, P=0.57). The average hFIX expression and average clotting times for all mice at each measured time point for both plasmids are shown in Figure 6c.
hFIX expression in mutant mouse plasma. Mice treated with pmGENIE3-hFIX and samples collected 1 (n=4), 4 or 5 (n=4) and 12 (n=4) days after UTMD. (a) Blot shown with hFIX recombinant protein positive control. Mut–C, untreated mutant plasma negative control; WT–C, untreated wild-type plasma negative control. Goat anti-mouse serum albumin antibody was used as a loading control. (b) Densitometric analysis of pmGENIE3-hFIX expression relative to mouse anti-serum albumin loading control.
hFIX expression in mutant mouse plasma. Mice treated with pZY53-hFIX and samples collected 1 (n=4), 4 or 5 (n=3) and 12 (n=3) days after UTMD. (a) Blot shown with hFIX recombinant protein positive control. Mut–C, untreated mutant plasma negative control; WT –C, untreated wild-type plasma negative control. Goat anti-mouse serum albumin antibody was used as a loading control. (b) Densitometric analysis of pZY53-hFIX expression relative to mouse anti-serum albumin loading control.
Relative hFIX expression compared with clotting times. (a) Relative hFIX expression versus clotting time for mice treated with pmGENIE3-hFIX. (b) Relative hFIX expression versus clotting time for mice treated with pZY53-hFIX. (c) Average hFIX expression and clotting times for each treatment time point for pmGENIE3-hFIX and pZY53-hFIX.
hFIX activity measured by APTT in treated FIX (−/−) mouse plasma
FIX-specific APTT assays were performed to assess the coagulant activity of citrated plasma from mice (Figure 7). Untreated citrated plasma was collected and analyzed from wild-type (n=22) and mutant (n=6) mice to establish control values for the assay (Table 1). Untreated FIX knockout plasma had a prolonged APTT time (136.1±12.1 s) and reduced clotting activity (<1%) compared with wild-type (48.1±3.4 s, ~100%) mice (P<0.0001, comparing mutant and wild-type mice). Pooled wild-type mouse plasma (n=10 mice) was serially diluted to generate an APTT standard curve (Supplementary Figure 2). The percentage of hFIX activity was extrapolated from the APTT standard curve.
APTT values for UTMD hFIX-treated mutant mice. Average clotting times for three time points tested for each group of mutant mice treated with pZY53-hFIX or pmGENIE3-hFIX by UTMD (n=3–4 mice per group, as indicated). Dashed lines indicate baseline average clotting times for untreated wild-type and mutant mice. One mutant control mouse (indicated by open square) was treated with pZY53-luc plasmid only by UTMD and APTT test was performed on plasma 1 day after UTMD as a negative control for hFIX. P=0.001 and P=0.02 for pZY53-hFIX, P=0.012 and P=0.001 for pmGENIE3-hFIX days 4–5 and 12 after UTMD, respectively.
The average clotting times obtained from mutant mice treated with pZY53-hFIX and pmGENIE3-hFIX are shown in Table 1 and Figure 7. No significant differences were observed in the plasma from mice treated with either plasmid 1 day after UTMD compared with untreated mutant controls. However, there was a significant reduction in the average clotting times for both hFIX plasmids in mice evaluated days 4 or 5 after UTMD compared with untreated mutant control mice (P=0.02 and P=0.001 for pZY53-hFIX and pmGENIE3-hFIX, respectively). Mice treated with pZY53-hFIX (n=3) displayed an average clotting time of 97±3 s that corresponds to ∼4.2% wild-type FIX activity 4 to 5 days after treatment compared with the average time of 127±23 s (<1.5% FIX activity) that was observed 1 day after UTMD. Mice treated with pmGENIE3-hFIX (n=4) had an average clotting time of 111±12 s and ∼2.7% wild-type FIX activity 4 to 5 days after UTMD compared with the average clotting time of 139±11 s (<1% FIX activity) observed 1 day after treatment. Importantly, a continued reduction in clotting time to 105±6 s and ~3% FIX activity was evident in pmGENIE3-hFIX-treated plasma samples (from n=4 mice) collected 12 days after UTMD. Clotting times in mice treated with pZY53-hFIX showed an increase to 108±17 s (corresponding with a slight decrease in activity to ∼3%) 12 days after treatment, but still improved compared with untreated mutant controls (P=0.001).
Evaluation of liver toxicity in treated FIX (−/−) mouse plasma
We saw an inconsistent increase in plasma alanine transaminase (ALT) levels early after UTMD for the pZY53-hFIX plasmid (58±16 U l−1 at 1 day, 68±17 U l−1 at 4 days and 43±9 U l−1 at 12 days) and a more consistent increase for pmGENIE3-hFIX (between 80 and 90 for all three time points) (Figure 8 and Supplementary Table 1, values are shown±s.e.m.). Overall, the increase in ALT, and some of the histology, suggests damage from the procedure, but we are not able to distinguish between an immune-mediated process, presumably to the hFIX, or direct ultrasound-mediated damage. Changes in ALT activity after UTMD delivery of pZY53-hFIX or pmGENIE3-hFIX were not statistically significant compared with each other or controls (P=0.75, P=0.37 and P=0.37 for mice treated with pZY53-hFIX compared with control groups at days 1, 4 or 5 and 12 after UTMD and P=0.27, P=0.28 and P=0.27 for mice treated with pmGENIE3-hFIX compared with control groups at days 1, 4 or 5 and 12 after UTMD).
ALT assay on treated mutant mouse plasma. Average ALT levels 1, 4 or 5 and 12 days after UTMD treatment with pZY53-hFIX (n=4 for day 1 and n=3 for days 4 or 5 and 12 time points) or pmGENIE3-hFIX (n=4 per time point). Untreated wild-type, mutant and pZY53-luc-treated mouse plasma served as controls (n=1 per control group).
Long-term evaluation of clotting time in treated FIX (−/−) mice
APTT assays were performed on plasma samples collected 160 days after UTMD treatment from a set of FIX (−/−) mice treated with pmGENIE3-hFIX (n=3). A statistically significant reduction in average clotting time was observed in the FIX (−/−)-treated mice (116.4±9.3 s) compared with plasma samples tested from untreated mutant control mice (n=6, 136.1±12.1 s, P=0.044). These data are shown together with the results obtained from the previous APTT experiments performed on plasma collected from pmGENIE3-hFIX-treated FIX (−/−) mice at days 1, 4 or 5 and 12 after UTMD (Figure 9 and Supplementary Table 2).
Long-term APTT evaluation in mutant mice treated with pmGENIE3-hFIX. Open circles indicate average clotting times in mice 1, 4 or 5, 12 and 160 days after treatment with pmGENIE3-hFIX (n=3–4 mice per group, as indicated). Dashed lines indicate baseline average clotting times for untreated wild-type (n=22, black triangle) and mutant (n=6, black square) mice. P=0.012, P=0.001 and P=0.044, days 4 or 5, 12 and 160 after UTMD, respectively, when compared with untreated mutant controls.
Discussion
The goal of this study was to examine the potential of UTMD to deliver nonviral hFIX vectors to the livers of mice with hemophilia B. We used a liver-specific hFIX expression cassette that had been shown to achieve therapeutic levels in mouse plasma when delivered by hydrodynamic tail vein injection.22 UTMD is an alternative method for gene delivery that could be clinically relevant in humans and is minimally invasive, allowing the option of repeated treatment. Miao et al.19 evaluated an invasive variant of UTMD to deliver hFIX expression plasmid to wild-type mice in a surgically exposed liver with direct infusion into the hepatic vein. This produced >1% hFIX activity in the plasma of these mice, and we hoped to achieve at least similar levels with our noninvasive strategy that is based on optimized sonographic parameters for UTMD to the murine liver.15
The murine FIX knockout model differs from the human disease in that the mice do not have any circulating levels of FIX, whereas many patients with hemophilia B have circulating levels of defective FIX. Perhaps for this reason patients with hemophilia B rarely develop neutralizing antibodies to FIX treatment (only 2–3% develop inhibitors), unlike the FIX (−/−) mice that commonly develop neutralizing antibodies to exogenously delivered FIX.3, 23
We established a reproducible FIX-specific method for testing the PTT clotting times in mice. Our clotting times for mutant mice were significantly prolonged and were consistent with previous reports; however, our values for wild-type mice (ranging from 42 to 52 s) were slightly longer than the 30–40 s range reported by the Jackson Laboratory phenome database for C57Bl/6 mice. We tentatively attribute this to the blood collection methods (retro-orbital versus cardiac puncture) and use of a manually operated hemostasis analyzer.
Some procedural death in knockout mice occurred shortly after performing UTMD. This seems likely to have been a result of the method of cardiac injection and would not be expected to occur in larger animals where more conventional intravenous access can be easily achieved. This is in contrast to the relative safety with which intracardiac injection can be used in wild-type mice.15, 16
Our histological evaluation of hFIX expression by immunofluorescence revealed transfection of hepatocytes predominantly in the left and medial liver lobes, with more transfection in hepatocytes near vessels (Figures 2c and d, for pZY53-hFIX; Figure 3c, for pmGENIE3-hFIX; and Supplementary Figures 3). This observation is consistent with our previous findings of luciferase expression from reporter vectors delivered to the mouse liver by UTMD. An increase in tissue damage including regions of hemorrhage with extravasation of red blood cells and coagulative necrosis was observed in several of the treated livers, particularly in samples analyzed 1 day after treatment with either plasmid (Figure 3d). We did not observe tissue damage in our previous studies in wild-type mice using the same UTMD parameters. We attribute the increase in tissue damage to an increased vulnerability to the effects of acoustic exposure and bleeding in the knockout mice. An ALT assay was performed to additionally assess liver damage in the treated mice. Miao et al.19 observed transient increases in ALT levels attributed to the effects of acoustic exposure in wild-type mice transfected with pZY53-hFIX by UTMD (using intrahepatic injections) when acoustic pressures of >2 MPa were applied. Although we observed increases in ALT levels in treated FIX (−/−) mice (Figure 8 and Supplementary Table 1), these changes varied substantially among animals and were not statistically significant compared with untreated and pZY53-luc treated control samples. The evidence of hepatic damage with acoustic peak negative pressures in the normal clinical range of 1.6–1.7 MPa suggests that contrast bubbles should be used with special care in hemophiliac patients.
We were able to detect hFIX expression in the plasma of mutant mice after UTMD by western blotting, and confirmed that there was no detectable hFIX expression in untreated mutant and wild-type control plasma (Figure 4) and minimal hFIX expression in the same controls shown in Figure 5 (perhaps a result of contamination from the positive control lane), demonstrating that hFIX was secreted by transfected hepatocytes into the blood. We observed a significant shortening of coagulation times (P=0.001 and P=0.012 for pZY53-hFIX and pmGENIE3-hFIX, respectively) in mice treated with pZY53-hFIX and pmGENIE3-hFIX 4 to 5 days after treatment. A persistent reduction in clotting times (P=0.02 and P=0.001 for pZY53-hFIX and pmGENIE3-hFIX, respectively) was observed in all four mice treated with pmGENIE3-hFIX and two of the three mice treated with pZY53-hFIX that were analyzed 12 days after treatment. FIX activity correlated to ∼4.5% and 2.5% using our standard curve for the average clotting times observed 4 to 5 days after treatment with pZY53-hFIX and pmGENIE3-hFIX, respectively. Clotting times in mice tested 12 days after UTMD remained virtually the same for pZY53-hFIX treatments (with a significant increase in one of the three treated mice) and demonstrated a slightly attenuated increase in FIX activity (~3%) for mice treated with pmGENIE3-hFIX (Figure 7). Miao et al.22 observed long-term hFIX expression (in wild-type mice) that achieved therapeutic levels (0.5–2.0 μg ml−1) with pZY53-hFIX plasmid delivered to the liver by hydrodynamic injection. Substantially lower levels were obtained using UTMD (up to 63 ng ml−1).19 These variations reflect the lower transfection efficiency intrinsic to UTMD compared with the more efficient hydrodynamic injection method.
We have previously shown that pmGENIE3-mediated delivery of reporter constructs to the liver produce durable expression after UTMD because of the genomic integration capacity of the transposon.15 In those studies, initial reporter activity was higher with a conventional plasmid, as in the present study, with both increased transfection efficiency and increased expression. Perhaps this is because of the smaller size of conventional plasmids. That earlier study also showed long-term expression of a reporter with pmGENIE. In the present study we have shown that expression from this plasmid can produce detectable and physiologically active hFIX, only slightly less robustly than the conventional plasmid. We also show that long-term improvement in clotting time with this strategy is feasible. Importantly, DNA transposon technology can be used to mediate transgene integration at specific loci (TTAA tetranucleotide sequences for the piggybac transposon) in cells targeted by UTMD. However, the actual integration patterns throughout the chromosome are not as specific, presenting important safety considerations for their use in gene therapy studies because of potential risks for insertional mutagenesis, gene silencing and/or dysregulation of nearby genes.9 The pmGENIE plasmid used in these studies utilizes a helper-independent system that encodes the piggyBac transposase (pBt) and transposon elements in the same construct and contains a transposase self-inactivation mechanism that may enhance the overall safety profile of the vector.13
Overall, the blood coagulation analyses show that even in this initial proof-of-principle study, one can obtain FIX activity that would be expected to ameliorate the bleeding diathesis in hemophilia B. We were able to demonstrate an average reduction in clotting time of ~39 and ~25 s (from the average untreated mutant control APTT value) at 4 to 5 days after mice were treated with pZY53-hFIX and pmGENIE3-hFIX, and an average reduction of ~18 and 31 s at 12 days after treatment with pZy53-hFIX and pmGENIE3-hFIX, respectively. However, in humans, an APTT value of >80 s is still associated with spontaneous bleeding, and thus increased transfection would be required for a therapeutic benefit. This could be achieved with a larger dose and more comprehensive insonation effort during an initial treatment, or taking advantage of the noninvasive nature of UTMD, repeated treatments to titrate the desired effect on clotting parameters. Repeated dosing, perhaps of distinct areas of the much larger human liver, might also help to minimize the cumulative hepatic damage of the procedure. An important limitation of the present study is the intracardiac approach to delivery of the bubbles. This works well in wild-type mice, and can be repeated safely. With the far more fragile FIX-deficient mice we were not able to successfully repeat the treatment and test the expected augmentation of expression from larger or repeated dosing.
Studies in larger animals will determine how the substantial differences in depth of field, hepatic anatomy and more gradual intravenous infusion may affect transfection efficiency and FIX expression. UTMD will be a useful way to evaluate new gene therapy vectors in FIX-deficient mice, and could be part of a new approach to titratable hepatic gene therapy in humans.
Materials and methods
Plasmids
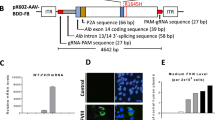
The pZY53-hFIX conventional plasmid was a generous gift from MA Kay (Stanford University, Palo Alto, CA, USA) and contains the apolipoprotein E enhancer/α1-antitrypsin liver-specific promoter (ZY53) with a hepatocyte control region encoding the human Factor IX complementary DNA (cDNA), as previously described.24 The pmGENIE3-hFIX plasmid was created by S Moisyadi (University of Hawaii at Manoa, Honolulu, HI, USA) and contains a cytomegalovirus-early-enhancer/chicken β-actin and β-globin intron promoter (CAG)-driven mouse-codon optimized piggyBac transposase (pBt) and the liver-specific promoter and hFIX cDNA from pZY53-hFIX between the 5′ and 3′ terminal repeat elements. The 3′ terminal repeat element of the transposon is situated in an intron of the pBt gene to result in truncation and enzymatic inactivation of the pBt gene upon transposition.13 The pZY53-luc plasmid is a liver-specific reporter constructed in our lab using the apolipoprotein E enhancer/α1-antitrypsin cDNA from pZY53-hFIX to replace the cytomegalovirus promoter in the pcDNA3-luc plasmid (Addgene Inc., Cambridge, MA, USA).
Preparation of microbubbles
Lipid-stabilized microbubbles were prepared as previously described using a stock solution of 200 mg of DPCC (DL-α-phosphatidylcholine, dipalmitolyl), 50 mg of DPPE (DL-α-phosphatidylethanolamine, dipalmitolyl) (both Merck KGaA, Darmstadt, Germany) and 1 g glucose mixed with phosphate-buffered saline to a final volume of 10 ml.16 The mixture was heated and agitated in a boiling water bath for 30 min and stored at 4 °C. Then, 250 μl of the cationic microbubble stock solution was warmed to 40 °C and added to 50 μl glycerol and 200 μl 1 × phosphate-buffered saline. Perfluoropropane gas was added to replace the microtube air space and the solution was mechanically mixed for 20 s in a Vialmix dental amalgamator (Lantheus Medical Imaging, North Billerica, MA, USA) and 500 μg of purified plasmid DNA dissolved in TE buffer was added. The DNA-bound microbubble solution was diluted with 1 × phosphate-buffered saline to a 1 ml final volume that was kept on ice and mixed by inversion before in vivo delivery. Using this protocol, ∼25 μg of plasmid DNA is delivered per 50 μl of injectate. This protocol has been previously determined to produce microbubbles with an average size of 2.1±0.9 μm and a concentration of ∼2.1±0.4 × 109 bubbles per ml as measured using a Beckman-Coulter Multisizer 3 (Brea, CA, USA).15, 25
Characterization of FIX (−/−) mice
All animal research was in compliance with ethical regulations and approved by the institutional animal care and use committee at the University of Hawaii.
Mice homozygous for a targeted (knockout) mutation in the factor IX gene, FIX(−) (F9tm1Dws, Stock No. 004303, The Jackson Laboratory) and wild-type C57BL/6 J (Stock No. 000664) were bred for these experiments. The mutated allele was generated by removing exons 1–3 of the factor IX gene.26 We confirmed deletion of the gene, RNA and protein for mouse FIX (data not shown).
FIX (−/−) mice husbandry and genotyping
Ear clipping was used to genotype the FIX (−/−) mice to facilitate hemostasis. PCR genotyping used the following primers: wild type forward, 5′-TGGAAGCAGTATGTTGGTAAGC-3′, common; mutant forward, 5′-AACAGGGATAGTAAGATTGTTCC-3′; and common (reverse), 5′-TCCTGTCATCTCACCTTGCTC-3′ (Supplementary Table 3). The deleted allele produced a fragment of ~550 bp, and the wild-type ~320 bp.
UTMD-mediated delivery of human FIX
The 8-week-old FIX (−/−) mice were anesthetized intraperitoneally with 100 mg kg−1 ketamine and 5 mg kg−1 xylazine. Under ultrasound guidance 50 μl of either a pZY53-hFIX or pmGENIE3-hFIX with pZY53-luc DNA-loaded microbubble solution was injected into the left ventricle of the heart (n=10 mice for pZY53-hFIX and n=15 for pmGENIE3-hFIX), as previously described.16 Three additional mice were included in the pmGENIE3-hFIX treatment group (n=15) for the long-term FIX evaluation. The microbubble bolus was visualized using a 30 MHz ultrasound transducer (VisualSonics 2100, transducer model RMV707B, Toronto, ON, Canada). Immediately following the injection, microbubble destruction was carried out for 2 min with a 1 s on, 2 s off pulsing time using a second, low-frequency 1.0 MHz unfocused transducer (General Electric, Chicago, IL, USA, model 025J19, 1.0 MHz/0.25”) that was traversed over the liver to obtain destruction of bubbles across this region. The ultrasound was administered to the liver in burst mode with an acoustic working frequency of 1.0 MHz, a pulse repetition period of 72 ms every 20 cycles producing an 18 μs pulse duration and a pulse repetition frequency of 13.9 Hz. These settings produced an acoustic peak negative pressure of 1.6–1.7 MPa in calibration studies. Liver and blood samples collected from untreated mutant mice served as controls for the histology, coagulation and liver toxicity studies. An additional set of mutant mice (n=3) were given intraventricular injections of equivalent doses of pZY53-luc plasmid only (no hFIX delivery) with microbubbles and were otherwise treated identically. These mice served as controls for the H&E histological studies to compare the hepatic effects of a hFIX-expressing versus non-hFIX liver-specific plasmid. Mice recovered with supplemental oxygen on a heating pad.
The samples sizes used in the current study were determined from outcomes observed in previous UTMD studies performed in our lab (using the same ultrasound parameters) with wild-type mice and conventional and pmGENIE-based reporter plasmids with liver-specific promoters. All pZY53-hFIX and pmGENIE3-hFIX UTMD-treated mutant mice were included in the reported analyses. Mutant mice were randomly allocated into either UTMD experimental treatment (pZY53-hFIX or pmGENIE3-hFIX plasmids) or nontreatment control groups. Specific methods of randomization and investigator blinding for the animal group allocation were not utilized.
In vivo bioluminescence
Bioluminescence imaging using the Xenogen in vivo imaging system (Caliper Life Sciences, Hopkinton, MA, USA) was used to detect transfected luciferase resulting from the co-delivery of pZY53-luc with either hFIX plasmid. Images were obtained the day after UTMD-mediated transfection to evaluate hepatic transfection. Briefly, mice were injected intraperitoneally with 150 mg kg−1 of the luciferase substrate D-luciferin diluted in sterile phosphate-buffered saline, followed by anesthesia 3 min later. Biodistribution of the D-luciferin substrate proceeded for 10 min before full-body in vivo imaging system image scans were obtained using a 10-min acquisition time. The in vivo imaging system software was used to measure the luminescent region of interest as the maximum photons per s per cm2 per steradian.
APTT assay
An APTT assay was developed for these experiments based upon previous studies with FIX (−/−) mice.23, 26, 27 Mouse plasma was prepared by collecting 9 volumes (450 μl) of blood in 1 volume (50 μl) of 0.109 M (3.2%) trisodium citrate anticoagulant prepared in water. Plasma was prepared by centrifugation for 15 min at 2000 g at 4 °C. FIX APTT assays were performed using a Start Hemostasis Analyzer (Diagnostica Stago Inc., Mount Olive, NJ, USA). For the APTT assay, 50 μl of APTT reagent, 50 μl of human factor IX-deficient plasma (Diagnostica Stago Inc., Cat. Nos. 00595 and 00724), and 50 μl of a 1:5 dilution of the citrated sample mouse plasma was incubated at 37 °C for 3 min. The time required for clot formation was measured after the addition of 50 μl of 25 mM CaCl2. In order to reduce artifactual FIX procoagulant activity in mutant mouse samples, 5 μl of untreated KO mouse plasma was added to each standard for the clotting assay.3, 23 Standards were prepared by diluting pooled C57Bl/6 wild-type mouse plasma (n=10 mice) 1:5 in Owren Koller buffer (acetate saline pH=7.35) (Diagnostica Stago Inc., Cat. No. 00360). The initial 1:5 dilution served as the reference for 100% activity and was serially diluted to obtain a standard curve.
Bleeding time assay
We compared bleeding time in wild-type and mutant mice. A half centimeter of the tail was amputated, and the tail was then positioned in a 0.6 ml microtube where blood was collected for 5 min. Tail clip wounds were cauterized and the volume of blood was measured (Supplementary Figure 1).28
Detection of hFIX by western blotting
Western blot assays were conducted on mouse plasma samples collected from control and mutant mice treated with the hFIX vectors using a monoclonal anti-human Factor IX antibody (Haematologic Technologies, Essex Junction, VT, USA, Cat. No. AHIX-5041) and an Alexa Fluor 568 fluorescent dye conjugate (Life Technologies, Carlsbad, CA, USA, goat anti-mouse, Cat. No. A-21124). Citrated plasma samples were subjected to electrophoresis through 12% polyacrylamide gels, and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA; Cat. No. IPVH00010). After blocking with 5% nonfat dried milk the blots were incubated overnight with primary antibody diluted 1:2000, and then fluorescent secondary antibody diluted 1:2000. The blots were washed and then imaged using a Typhoon FLA 9410 laser scanner (GE Healthcare Life Sciences, Pittsburgh, PA, USA). The hFIX immunoblots were stripped and reprobed with the goat anti-mouse serum albumin antibody (Bethyl Laboratories Inc., Montgomery, TX, USA, Cat. No. A90-2394) at a 1:1000 dilution. The membranes were washed and incubated with a donkey anti-goat 568 Alexa Fluor 568-conjugated secondary antibody diluted 1:2000. Densitometry analyses of western blot bands were evaluated using ImageJ software (NIH, Bethesda, MD, USA).
Histology
Mice were killed by CO2 asphyxiation and tissue was formalin fixed by perfusion through the left ventricle. Whole fixed livers were excised and dissected into the left, medial, right and caudate lobes. Fixed liver lobes were further incubated in formalin and transferred to 70% ethanol before paraffin embedding and sectioning. Immunofluorescence and H&E staining was performed on sections for each liver lobe for each mouse for evaluation of hFIX expression and liver damage, respectively. For immunofluorescence, sections were subjected to antigen retrieval by a 30-min incubation in a 95 °C citrate solution, pH 6.1 (Dako, Carpinteria, CA, USA, Cat. No. S170084-2). After blocking, a 1:400 dilution of a polyclonal goat anti-hFIX primary antibody (Affinity Biologicals, Ancaster, ON, Canada, Cat. No. GAFIX-AP) was applied followed by incubation in a 1:400 dilution of donkey anti-goat Alexa Fluor 488 secondary antibody (Life Technologies, Cat. No. A-11055). The 4′-6-Diamidino-2-phenylindole (DAPI) fluorescent stain was used as a fluorescent probe for nuclei. Fluorescent microscopy was used to evaluate liver cell hFIX transgene expression and localization. The human FIX antibody can discriminate exogenous human FIX from endogenous mouse FIX (Supplementary Figure 3).
ALT activity assay
ALT activity was evaluated in treated mutant mouse plasma and compared with mutant control plasma using a commercial kit (Cayman Chemical Company, Ann Arbor, MI, USA, Cat. No. 700260) following the manufacturer’s protocol. For a positive control, 20 μl of a 1.0 IU ml−1 solution of ALT enzyme (from porcine heart) was used in place of the sample. The limit of detection of the assay is 0.006 U ml−1.
Statistical analysis
Data were analyzed using the GraphPad Prism program (Version 5.0b, La Jolla, CA, USA). Mean values were calculated for experimental groups and error bars indicate±s.d. or s.e.m. as noted. Student’s t-test (two-sided) was used to analyze differences in hFIX expression between groups and analysis of variance was used for multiple group comparisons. P-value of <0.05 was considered to be statistically significant.
References
Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med 1999; 5: 64–70.
Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997; 16: 270–276.
Monahan PE . Factor IX: insights from knock-out and genetically engineered mice. Thromb Haemost 2008; 100: 563–575.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365.
Miao CH . Advances in overcoming immune responses following hemophilia gene therapy. J Genet Syndr Gene Ther 2011; S1: 007.
Nathwani AC, Reiss UM, Tuddenham EG, Rasales C, Chowdary P, McIntosh J et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014; 371: 1994–2004.
Barzel A, Paulk NK, Shi Y, Huang Y, Chu K, Zhang F et al. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature 2015; 517: 360–364.
Gracey Maniar LE, Maniar JM, Chen ZY, Fire AZ, Kay MA . Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther 2013; 21: 131–138.
Kay MA . State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet 2011; 12: 316–328.
Keravala A, Chavez CL, Hu G, Woodard LE, Monahan PE, Calos MP . Long-term phenotypic correction in factor IX knockout mice by using PhiC31 integrase-mediated gene therapy. Gene Therapy 2011; 18: 842–848.
Keravala A, Lee S, Thyagarajan B, Olivares EC, Gabrovsky VE, Woodward LE et al. Mutational derivatives of PhiC31 integrase with increased efficiency and specificity. Mol Ther 2009; 17: 112–120.
Mátés L, Chuah MK, Belay E, Jerchow B, Manoj N, Acosta-Sanchez A et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet 2009; 41: 753–761.
Urschitz J, Kawasumi M, Owens J, Morozumi K, Yamashiro H, Stoytchev I et al. Helper-independent piggyBac plasmids for gene delivery approaches: strategies for avoiding potential genotoxic effects. Proc Natl Acad Sci USA 2010; 107: 8117–8122.
Woodard LE, Hillman RT, Keravala A, Lee S, Calos MP . Effect of nuclear localization and hydrodynamic delivery-induced cell division on phiC31 integrase activity. Gene Therapy 2010; 17: 217–226.
Anderson CD, Urschitz J, Khemmani M, Owens JB, Moisyadi S, Shohet RV et al. Ultrasound directs a transposase system for durable hepatic gene delivery in mice. Ultrasound Med Biol 2013; 39: 2351–2361.
Walton CB, Anderson CD, Boulay R, Shohet RV . Introduction to the ultrasound targeted microbubble destruction technique. J Vis Exp 2011; 52: 2963.
Chen ZY, He CY, Ehrhardt A, Kay MA . Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther 2003; 8: 495–500.
Lindner JR . Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 2004; 3: 527–532.
Miao CH, Braymann AA, Loeb KR, Ye P, Zhou L, Mourad P et al. Ultrasound enhances gene delivery of human factor IX plasmid. Hum Gene Ther 2005; 16: 893–905.
Fujii H, Li SH, Wu J, Miyagi Y, Yau TM, Rakowski H et al. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J 2011; 32: 2075–2084.
Chen ZY, He CY, Kay MA . Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther 2005; 16: 126–131.
Miao CH, Thompson AR, Loeb K, Ye X . Long-term and therapeutic-level hepatic gene expression of human factor IX after naked plasmid transfer in vivo. Mol Ther 2001; 3: 947–957.
Jin DY, Zhang TP, Gui T, Stafford DW, Monahan PE . Creation of a mouse expressing defective human factor IX. Blood 2004; 104: 1733–1739.
Miao CH, Ohashi K, Patijn GA, Meuse L, Ye X, Thompson AR et al. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol Ther 2000; 1: 522–532.
Bekeredjian R, Grayburn PA, Shohet RV . Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol 2005; 45: 329–335.
Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW . A coagulation factor IX-deficient mouse model for human hemophilia B. Blood 1997; 90: 3962–3966.
Wang L, Zoppè M, Hackeng TM, Griffin JH, Lee KF, Verma IM . A factor IX-deficient mouse model for hemophilia B gene therapy. Proc Natl Acad Sci USA 1997; 94: 11563–11566.
Kung SH, Hagstom JN, Cass D, Tai SJ, Lin HF, Stafford DW et al. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood 1998; 91: 784–790.
Acknowledgements
We thank Miyoko Bellinger and Kristen Ewell from the John A. Burns School of Medicine (JABSOM) Histology Core for histology processing support, the Imaging Core for microscopy resources and Aaron Tuia from the JABSOM Mouse Phenotyping Core for animal husbandry support. We also thank Mark Kay from Stanford University for providing us with the cDNA for the apolipoprotein E enhancer/α1- antitrypsin (hATT) promoter and human factor IX gene. This work was supported by NIH Grants HL080532, HL073449 and GM103341 and their supplements (to RVS), MD007601 (to CBW), GM103457 (to SM), and Western Affiliate AHA Predoctoral Fellowship 12040462 (to CDA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Stefan Moisyadi is the owner of Manoa BioSciences, a start-up company out of the University of Hawaii. Manoa BioSciences holds the rights and patent for the pmGENIE construct.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Anderson, C., Moisyadi, S., Avelar, A. et al. Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice. Gene Ther 23, 510–519 (2016). https://doi.org/10.1038/gt.2016.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.23
This article is cited by
-
Tissue Targeting and Ultrasound-Targeted Microbubble Destruction Delivery of Plasmid DNA and Transfection In Vitro
Cellular and Molecular Bioengineering (2020)
-
Nonviral ultrasound-mediated gene delivery in small and large animal models
Nature Protocols (2019)
-
Synthetic materials at the forefront of gene delivery
Nature Reviews Chemistry (2018)