Abstract
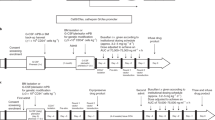
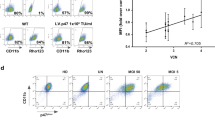
Insertional mutagenesis represents a serious adverse effect of gene therapy with integrating vectors. However, although uncontrolled activation of growth-promoting genes in stem cells can predictably lead to oncological processes, this is far less likely if vector transcriptional activity can be restricted to fully differentiated cells. Diseases requiring phenotypic correction only in mature cells offer such an opportunity, provided that lineage/stage-restricted systems can be properly tailored. In this study, we followed this reasoning to design lentiviral vectors for the gene therapy of chronic granulomatous disease (CGD), an immune deficiency due a loss of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in phagocytes, most often secondary to mutations in gp91phox. Using self-inactivating HIV1-derived vectors as background, we first expressed enhanced green fluorescent protein (eGFP) from a minimal gp91phox promoter, adding various natural or synthetic transcriptional regulatory elements to foster both specificity and potency. The resulting vectors were assessed either by transplantation or by lentiviral transgenesis, searching for combinations conferring strong and specific expression into mature phagocytic cells. The most promising vector was modified to express gp91phox and used to treat CGD mice. High-level restoration of NADPH activity was documented in granulocytes from the treated animals. We propose that this lineage-specific lentiviral vector is a suitable candidate for the gene therapy of CGD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Winkelstein JA, Marino MC, Johnston Jr RB, Boyle J, Curnutte J, Gallin JI et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000; 79: 155–169.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409.
Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med 2010; 16: 198–204.
Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 2002; 13: 803–813.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009; 326: 818–823.
Baum C . Insertional mutagenesis in gene therapy and stem cell biology. Curr Opin Hematol 2007; 14: 337–342.
Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol 2004; 2: E234.
Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 2009; 17: 1919–1928.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006; 24: 687–696.
Skalnik DG, Dorfman DM, Perkins AS, Jenkins NA, Copeland NG, Orkin SH . Targeting of transgene expression to monocyte/macrophages by the gp91-phox promoter and consequent histiocytic malignancies. Proc Natl Acad Sci USA 1991; 88: 8505–8509.
Asante EA, Gowland I, Linehan JM, Mahal SP, Collinge J . Expression pattern of a mini human PrP gene promoter in transgenic mice. Neurobiol Dis 2002; 10: 1–7.
Choi T, Huang M, Gorman C, Jaenisch R . A generic intron increases gene expression in transgenic mice. Mol Cell Biol 1991; 11: 3070–3074.
He W, Qiang M, Ma W, Valente AJ, Quinones MP, Wang W et al. Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther 2006; 17: 949–959.
Ramezani A, Hawley TS, Hawley RG . Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood 2003; 101: 4717–4724.
Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG et al. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc Natl Acad Sci USA 2002; 99: 6883–6888.
Sauvain MO, Dorr AP, Stevenson B, Quazzola A, Naef F, Wiznerowicz M et al. Genotypic features of lentivirus transgenic mice. J Virol 2008; 82: 7111–7119.
Volkman DJ, Buescher ES, Gallin JI, Fauci AS . B cell lines as models for inherited phagocytic diseases: abnormal superoxide generation in chronic granulomatous disease and giant granules in Chediak-Higashi syndrome. J Immunol 1984; 133: 3006–3009.
Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 1995; 9: 202–209.
Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA . Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods 1995; 178: 89–97.
Malech HL . Progress in gene therapy for chronic granulomatous disease. J Infect Dis 1999; 179 (Suppl 2): S318–S325.
Maly FE, Nakamura M, Gauchat JF, Urwyler A, Walker C, Dahinden CA et al. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J Immunol 1989; 142: 1260–1267.
Santilli G, Almarza E, Brendel C, Choi U, Beilin C, Blundell MP et al. Biochemical correction of X-CGD by a novel chimeric promoter regulating high levels of transgene expression in myeloid cells. Mol Ther 2011; 19: 122–132.
Kustikova OS, Schiedlmeier B, Brugman MH, Stahlhut M, Bartels S, Li Z et al. Cell-intrinsic and vector-related properties cooperate to determine the incidence and consequences of insertional mutagenesis. Mol Ther 2009; 17: 1537–1547.
Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M et al. Resistance of mature T cells to oncogene transformation. Blood 2008; 112: 2278–2286.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010; 467: 318–322.
VandenDriessche T, Thorrez L, Naldini L, Follenzi A, Moons L, Berneman Z et al. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood 2002; 100: 813–822.
Grebe M, Gadea J, Steinmann T, Kientz M, Rahfeld JU, Salchert K et al. A conserved domain of the Arabidopsis GNOM protein mediates subunit interaction and cyclophilin 5 binding. Plant Cell 2000; 12: 343–356.
Charrier S, Dupre L, Scaramuzza S, Jeanson-Leh L, Blundell MP, Danos O et al. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Therapy 2007; 14: 415–428.
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D . Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997; 15: 871–875.
Charrier S, Stockholm D, Seye K, Opolon P, Taveau M, Gross DA et al. A lentiviral vector encoding the human Wiskott-Aldrich syndrome protein corrects immune and cytoskeletal defects in WASP knockout mice. Gene Therapy 2005; 12: 597–606.
Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D . Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002; 295: 868–872.
Acknowledgements
We thank L Rigonnot, L Khadam, their staff and the mothers at the Maternity ward of Hopital Louise Michel, Evry, France for providing umbilical cord blood samples. We thank J Dick for his support in performing experiments. We also thank L Naldini, S Li, P Salmon and KH Krause for the gifting of reagents, and M Garcia and G Tapia for help with the FACS studies. This work was supported by grants from the Swiss National Science Foundation and from the European Union (FP6 CONSERT and FP7 PERSIST projects).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Barde, I., Laurenti, E., Verp, S. et al. Lineage- and stage-restricted lentiviral vectors for the gene therapy of chronic granulomatous disease. Gene Ther 18, 1087–1097 (2011). https://doi.org/10.1038/gt.2011.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.65
Keywords
This article is cited by
-
A synthetic transcription platform for programmable gene expression in mammalian cells
Nature Communications (2022)
-
Lentiviral Vector Gene Therapy Protects XCGD Mice From Acute Staphylococcus aureus Pneumonia and Inflammatory Response
Molecular Therapy (2016)
-
Assembly PCR synthesis of optimally designed, compact, multi-responsive promoters suited to gene therapy application
Scientific Reports (2016)
-
Lentiviral Gene Therapy Using Cellular Promoters Cures Type 1 Gaucher Disease in Mice
Molecular Therapy (2015)
-
Dual-regulated Lentiviral Vector for Gene Therapy of X-linked Chronic Granulomatosis
Molecular Therapy (2014)