Abstract
Purpose: To characterize the clinical outcome of heterozygosity for COL3A1 null mutations in Ehlers-Danlos syndrome type IV, the vascular type.
Methods: We identified mutations that produced premature termination codons and resulted in nonsense-mediated messenger RNA decay in 19 families. We reviewed the clinical and family histories and medical complications in 54 individuals from these families with COL3A1 null mutations.
Results: Compared with individuals with missense or exon-skipping mutations, we found that life span was extended, the age of first complication was delayed by almost 15 years, and major complications were limited to vascular events. The families were ascertained after a complication in a single individual, but only 28% of relatives, some of whom had reached their seventies or eighties without incidents, had a complication and only 30% had minor clinical features of Ehlers-Danlos syndrome type IV
Conclusion: Null mutations have reduced penetrance compared with missense and splicing mutations, and the phenotype seems to be limited almost entirely to vascular events.
Similar content being viewed by others
Main
Ehlers-Danlos syndrome (EDS) type IV, known as the vascular type (OMIM# 130050), is a dominantly inherited disorder that results from mutations in the COL3A1 gene (OMIM# 120180). EDS type IV is characterized by the major complications of arterial and bowel rupture, uterine rupture during pregnancy, and the clinical features of easy bruising, thin skin with visible veins, and characteristic facial features. Because of these dramatic complications, mean life expectancy is shortened to <50 years.1
We have identified heterozygous COL3A1 mutations in 508 families, approximately 95% of which lead to the synthesis of abnormal type III procollagen molecules. Alterations in glycine codons that lead to substitutions for glycine residues in the triple helical domain of the proα1(III) chain of type III procollagen account for two thirds of identified mutations, and splicing mutations comprise most of the remainder.1,2 The remaining mutations include small genomic deletions and duplications, multiexon deletions, and more complex alterations. A total of 19 of the 508 families have mutations that lead to premature termination codons expected to result in nonsense-mediated messenger RNA (mRNA) decay of the encoded product of the mutant allele. This distribution of mutations is similar to that reported in the Database of Collagen Mutations.3 To date, no correlations have been identified between the nature of the missense mutations or splice-site alterations and the type or frequency of major complication,1 perhaps because almost all reported individuals have been ascertained as a result of complications of the disorder.
In most other fibrillar collagenopathies, production of half the amount of the encoded protein as a result of haploinsufficiency usually results in a milder phenotype than seen with missense or splicing mutations. For example, haploinsufficiency mutations in COL1A1 (OMIM# 120150) result in osteogenesis imperfecta (OI) type I, the mildest phenotype. Similar mutations in COL2A1 result in Stickler syndrome, rather than the more severe phenotypes seen with missense and exon skipping mutations. Mice heterozygous for a COL3A1 knockout do not develop arterial rupture4 but have histologic and mechanical changes of the artery wall,5 which suggests that in humans haploinsufficiency for COL3A1 might be expected to have a milder clinical picture than in individuals with missense or other mutations that alter type III collagen structure. In the initial set of EDS type IV families identified with heterozygosity for COL3A1 null mutations, the index patients had a clinical course similar to that seen in individuals with missense or splice-site mutations.2 However, testing of relatives determined that many affected family members survived beyond age 50 years without major complications (see data presented herein). One teenager, who was homozygous for a COL3A1 frameshift mutation that led to mRNA instability, died from complications similar to those seen in others with heterozygous missense mutations, but neither of her heterozygous parents had any evidence of such adverse effects, and there was no family history of similar complications.6
To understand the clinical consequences of heterozygosity for COL3A1 null mutations, we reviewed the clinical and family histories and medical complications of 19 index patients with null mutations and 35 of their relatives who had the same mutation. Compared with individuals with missense or exon-skipping mutations, we found that in the cohort with COL3A1 null mutations, life span was extended, the age of first complication was delayed, major complications were limited to vascular events, and penetrance was reduced.
MATERIALS AND METHODS
Study subjects
Eighteen of 19 index patients (95%) with identified COL3A1 null mutations were referred for evaluation after a major arterial event. The remaining index patient was referred because a parent died at an early age from an arterial event. Testing was completed on 54 relatives because of the perceived risk for vascular complications in individuals with these mutations.2 All relatives in whom a COL3A1 mutation was identified in the Collagen Diagnostic Laboratory were referred because of a positive family history; none of these relatives was tested because of a vascular complication. Three of the index patients included in this study (1, 2, and 8) were reported by Schwarze et al.2 (P1, P2, and P3 in that article). The remaining subject (P4) in that study is not included in this study because that mutation resulted in protein instability rather than mRNA instability, and it is not known whether mutations that interfere with chain association activate an unfolded protein response that may add an additional component to the response. Those with premature termination codons seem to activate only the nonsense-mediated mRNA decay pathway.
Biochemical and DNA sequencing studies
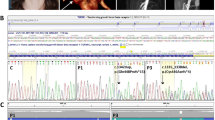
Dermal fibroblasts were obtained and cultured from 12 of the 19 index patients (but none of the relatives) referred to the Collagen Diagnostic Laboratory for collagen screening studies, and the synthesis of type III procollagen was studied as described previously.7 Blood samples were submitted from the remaining index patients and 54 of the relatives for clinical testing. DNA was extracted by standard procedures. The coding exons and flanking intron sequences of COL3A1 were amplified by PCR in 25 reactions using primer pairs based on the 2006 freeze of the Human Genome Sequence. The amplified fragments were sequenced by automated sequencing (primers and amplification and sequencing conditions are available on request). To test relatives, only the region of COL3A1 that contained the mutation in the family was amplified and sequenced, using the same amplification and sequencing primers used for mutation discovery.
Analysis of mutation outcome
Total cellular RNA was isolated by RNeasy Mini Kit (Qiagen) from cultured dermal fibroblasts from 12 of the index patients. In addition, nuclear RNA was isolated for index patient 12 as described elsewhere.8 Complementary DNA was synthesized using SuperScript II reverse transcriptase (Invitrogen, San Diego, CA). A region of the COL3A1 coding sequence was amplified that included the mutation, a second unique heterozygous variant, or a polymorphic site found to be heterozygous during genomic DNA sequence determination and then sequenced to determine the relative abundance of the products of the two COL3A1 alleles (Table 1).
Assignment of affected status
We identified 63 relatives with a 50% risk to have inherited or transmitted a null mutation found in the 19 families. We were able to test 54 of these relatives to determine whether they had their respective familial mutation. Of these, we found a mutation in 26 and did not find a mutation in 28. All those tested in whom the familial mutation was identified were designated as “affected” for the sake of this study. There were nine individuals among the 63 relatives who we could not test but to whom we assigned an affected status because they had a major arterial complication and a confirmed affected first-degree relative (6 subjects) or on the basis of obligate inheritance (3 subjects). Relatives were classified as unaffected if the results of testing excluded the diagnosis. We did not include in the analysis individuals for whom no clinical information was available, if clinical information was insufficient to make a clinical diagnosis, or if the individual was reported as healthy and not tested.
From clinical histories available at the time of diagnostic testing, we recorded the number and type of medical complications, the ages at which they occurred, the cause of and age at death, and minor clinical findings of EDS type IV,9 if noted. The age of testing (i.e., ascertainment) in the index patients was the age at which we confirmed the diagnosis (Table 2). For their relatives, the age of ascertainment was the age at which we identified the familial mutation in them (26 relatives), their last known age (three subjects), the age they were reported in the literature (one subject),10 or their age of death as recorded in the family history (five subjects).
Statistical analysis
We used Kaplan-Meier analysis (SPSS statistical software) to generate a survival curve and confidence intervals for the null cohort (Figure, Supplemental Digital Content 1, http://links.lww.com/GIM/A176); we included the age of death or the last known living age of each living subject. For a basis of comparison, we recalculated the Kaplan-Meier survival curves and confidence intervals for EDS type IV index patients and their relatives using the original data presented by Pepin et al.1 None of the subjects included in this study were in the previous study.
RESULTS
Mutations and mutation outcome
We identified four nonsense mutations, nine frameshift mutations that led to downstream premature termination codons, and six splice-site mutations that created frameshifts and downstream premature termination codons (Table 1). Cultured cells from eight of these individuals (Families 4, 6, 8, 10, 11, 12, 15, and 18) produced about half the normal amount of type III procollagen and no abnormal molecules, as predicted by the type of mutation. In cells from individuals in four of the families (1, 2, 3, and 9), we could not distinguish the amount of type III procollagen produced from the control, and no abnormality was detected in the mobilities of type III collagen chains.
In all families for which cultured fibroblasts were available, the mutation was shown to result in nonsense-mediated mRNA decay (Table 1). For the remainder, the effect was inferred from the predicted outcome of the other mutations.
Survival
The index patients were identified at a younger age than were their affected relatives (Table 2). A total of seven subjects died: two index patients (2/19; 10%) and five relatives (5/35; 14%). In the natural history study of individuals with EDS type IV by Pepin et al.,1 a similar proportion of index patients died (26/220; 11.8%), but a much larger proportion of relatives (105/199, 53%) were not alive at the time of ascertainment. Pepin et al.1 attributed the higher death rate of relatives to the method of ascertainment (using the family history records of younger index patients for diagnosis and inclusion). Using the same method of ascertainment, we found, unexpectedly, a lower death rate in relatives in this group than in those with other types of mutations (Fig. 1).
Distribution by age of individuals with COL3A1 null mutations and other types of mutations in COL3A1. The distribution is arranged in 5-year intervals. Each box contains the number of individuals at their last known age (numerator), the total number in the group (denominator), and the percentage of the total.
The ages of death were 31, 36, 38, 41, 53, 58, and 68 years in the patients who died in the null cohort. Despite the small sample size, there is a difference in survival between the groups (Figure, Supplemental Digital Content 1, http://links.lww.com/GIM/A176). A log-rank test of the Kaplan-Meier analysis confirmed a significant difference in survival (P < 0.005, χ2 value was 21.2, with a degree of freedom of 1).
All deaths in this study resulted from arterial dissection or rupture, compared with 79% of deaths from arterial complications, 10% from organ rupture, 7% from gastrointestinal rupture, and 4% other causes in the study by Pepin et al.1
Medical complications
At the time of ascertainment, 18 of 19 index patients (95%) and 10 of 35 relatives (28%) had had a single vascular complication (arterial dissection, aneurysm, or rupture). One index patient (5%) and 25 of 35 relatives (71%) had not had any type of vascular, bowel, or gastrointestinal complication at the time of ascertainment.
Arterial complications
Of the 18 index patients with arterial complications, 12 had had dissections (three carotid artery dissections, three abdominal aortic dissections, two thoracic aortic dissections, two renal artery dissections, one coronary artery dissection, and one unknown). Four of the index patients had an arterial aneurysm as their first complication (common hepatic artery aneurysm, abdominal aortic aneurysm, splenic artery aneurysm, and aneurysms at multiple locations), whereas the remaining two index patients had multiple arterial ruptures (celiac, superior mesenteric, hepatic, splenic, renal, iliac, and femoral) as their primary complication. Of the 10 relatives with arterial complications, three had dissections (thoracoabdominal aortic, extracranial vertebral, and internal carotid), three had arterial ruptures (two aortic ruptures and one femoral artery rupture after catheterization), one had a hepatic artery aneurysm, and the specific details were not available for the remaining three. Of the 25 relatives without a complication at the time of ascertainment, one individual had a normal abdominal ultrasound, but no others were studied by magnetic resonance imaging or computed tomography for evidence of arterial abnormalities.
Minor features of EDS type IV
One or more of the minor diagnostic criteria for the vascular type of EDS (as defined by Beighton et al.9) were reported as present in 41% of null subjects, with thin skin (16%), characteristic facial features (14%), joint laxity (12%), or unusual scarring (12%) being the most common (Fig. 2). In none of the patients with null mutations were friable tissue and poor wound healing reported, characteristics common in other individuals with EDS type IV. More than half of the null cohort (51%) was reported to have no minor clinical features of EDS type IV.
DISCUSSION
Our study indicates that most individuals with COL3A1 null mutations have a different clinical course than individuals with missense or splice-site mutations that result in the production of abnormal type III procollagen molecules. Compared with individuals with mutations that produce abnormal molecules, those with COL3A1 null mutations had an extended life span, the average age of first complication was delayed by almost 15 years, and primary complications were limited to vascular events (dissections, aneurysms, or ruptures).
Families with null mutations were ascertained at a later age than those with mutations that altered protein structure (44.4 years vs. 28.7 years). Although 18 of 19 of these families were ascertained after a vascular complication in a single individual, only 28% of relatives who had the same mutation reported a vascular complication. Thus, most relatives with COL3A1 null mutations did not have the complications associated with EDS type IV, and only a minority was thought to have even the minor clinical features of the condition.
One limitation of this study is that we relied on available medical history to determine whether individuals with the mutation had complications of EDS type IV. Because those without complications had no reason to suspect they were at risk before mutation characterization, they would not have had detailed imaging for aneurysms or undetected dissections in major vessels. Thus, we could not identify the true incidence of vascular complications in this group, only in those who were symptomatic. Additionally, we were not able to study individuals in these families who had no complications consistent with EDS type IV and no testing. This exclusion biases ascertainment toward those with complications (in some instances assumed to result because they had mutations). Finally, because the ascertainment of null families seems to be far below what we would expect, this study may overestimate the risk of arterial complications of heterozygosity for null mutations.
We remain uncertain why analysis of the proteins produced by the cultured cells is incompletely sensitive to the presence of null mutations; it may reflect the relative expression of the intact COL3A1 allele. The diminished sensitivity of protein-based studies in detection of COL3A1 mutations that result in premature termination codons suggests direct DNA analysis as the preferred mode of diagnostic testing. However, in rare instances, analysis of the outcome of some mutations, particularly those that affect splice sites, may be required to prove that the ultimate effect is to reduce mRNA stability. These studies can only be done in cultured cells.
Null mutations in genes that encode the dominant protein of a tissue, such as the collagen genes, often have a milder phenotype. In families with OI that results from mutations in the COL1A1 gene, almost 50% of affected individuals who we studied have haploinsufficiency mutations and have OI type I, the mildest OI phenotype (unpublished data). However, COL3A1 null mutations represent only 4% of mutations we have identified in that gene. Because null mutations in COL3A1 are likely to be as common or nearly as common as those in COL1A1, the penetrance of this class of mutation is lower than that of missense and splicing mutations. This suggests that there may be a second variant (perhaps the relative expression of the intact COL3A1 allele) that predisposes individuals with the primary mutation to develop arterial events. We are uncertain where the bulk of individuals with COL3A1 null mutations are to be found, but if the analogy with OI holds, then perhaps they are represented among individuals with late-onset arterial aneurysms. It is appropriate to launch a research study to determine the extent to which such mutations account for late-onset aneurysms and dissections.
In current practice, it is the presence of a family history of vascular or organ complication and the presence of at least some of the minor clinical features of EDS type IV that drives the decision to test for mutations in COL3A1. Our study suggests that lowering the threshold for such testing to include individuals with aneurysms or dissections at a later age and individuals with aneurysms or dissections and a positive family history of aneurysms or dissections may identify some of the individuals with this class of mutations. Finally, because ascertainment on the basis of clinical findings alone has a low yield, genetic testing for COL3A1 mutations should be offered to all first-degree relatives of an individual identified with a COL3A1 null mutation.
REFERENCES
Pepin M, Schwarze U, Superti-Furga A, Byers PH . Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000; 342: 673–680.
Schwarze U, Schievink WI, Petty E, et al. Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am J Hum Genet 2001; 69: 989–1001.
Dalgleish R . The human type I collagen mutation database. Nucleic Acids Res 1997; 25: 181–187.
Liu X, Wu H, Byrne M, Krane S, Jaenisch R . Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA 1997; 94: 1852–1856.
Cooper T, Zhong Q, Krawczyk M, et al. The haploinsufficient Col3a1 mouse as a model for vascular Ehlers-Danlos syndrome. Vet Pathol 2010; 47: 1028–1039.
Plancke A, Holder-Espinasse M, Rigau V, Manouvrier S, Claustres M, Van Kien PK . Homozygosity for a null allele of COL3A1 results in recessive Ehlers-Danlos syndrome. Eur J Hum Genet 2009; 17: 1411–1416.
Schwarze U, Goldstein JA, Byers PH . Splicing defects in the COL3A1 gene: marked preference for 5 ' (donor) splice-site mutations in patients with exon-skipping mutations and Ehlers-Danlos syndrome type IV. Am J Hum Genet 1997; 61: 1276–1286.
Schwarze U, Starman B, Byers P . Redefinition of exon 7 in the COL1A1 gene of type I collagen by an intron 8 splice-donor-site mutation in a form of osteogenesis imperfecta: influence of intron splice order on outcome of splice-site mutation. Am J Hum Genet 1999; 65: 336–344.
Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ . Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet 1998; 77: 31–37.
Khalique Z, Lyons OTA, Clough RE, et al. Successful endovascular repair of acute type B aortic dissection in undiagnosed Ehlers-Danlos syndrome type IV. Eur J Vasc Endovasc Surg 2009; 38: 608–609.
Acknowledgements
This work was supported, in part, by the Freudmann Fund for Translational Research in Ehlers-Danlos Syndrome and The Ehlers Danlos Type IV Research Fund at the University of Washington. The authors thank the families and their clinicians for making information available for the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.geneticsinmedicine.org).
Rights and permissions
About this article
Cite this article
Leistritz, D., Pepin, M., Schwarze, U. et al. COL3A1 haploinsufficiency results in a variety of Ehlers-Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet Med 13, 717–722 (2011). https://doi.org/10.1097/GIM.0b013e3182180c89
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3182180c89
Keywords
This article is cited by
-
Audit of Gastrointestinal Manifestations in Patients with Loeys–Dietz Syndrome and Vascular Ehlers–Danlos Syndrome
Digestive Diseases and Sciences (2021)
-
Vascular Ehlers-Danlos Syndrome Presenting as a Pulsatile Neck Mass: a Case Report and Review of Literature
Journal of General Internal Medicine (2018)
-
Heterogeneous nanomechanical properties of type I collagen in longitudinal direction
Biomechanics and Modeling in Mechanobiology (2017)
-
Gene panel sequencing in heritable thoracic aortic disorders and related entities – results of comprehensive testing in a cohort of 264 patients
Orphanet Journal of Rare Diseases (2015)
-
The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers–Danlos syndrome
European Journal of Human Genetics (2015)