Abstract
Aim
To investigate clinical presentation and genotypes in patients with simultaneous geographic atrophy (GA) and choroidal neovascularization (CNV) and to compare with patients with GA or CNV only.
Patients and methods
Twenty patients with combined CNV–GA and 154 CNV only and 154 GA only were chosen based on clinical exam and imaging. Six single-nucleotide polymorphisms (SNPs)—rs2274700 and rs1061170 (complement factor H), rs10490924 and rs11200638 (HTRA1/LOC387715), rs2230199 (C3), rs9332739 (C2)—were genotyped using the SNaPshot method. Chi-squared tests were used for genetic analysis.
Results
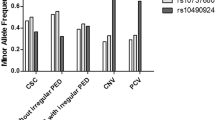
In patients with CNV–GA, GA progressed slowly and often preceded CNV. CNV presented as subretinal haemorrhage or fluid, with a sudden drop in visual acuity (VA). Comparing combined CNV–GA to GA and CNV only, patients with both had a higher frequency of at-risk alleles at both SNPs within the HTRA1 gene—rs10490924 (52.5%), rs11200638 (52.6%). Statistical significance was not achieved. CNV–GA patients had no protective alleles at SNP rs9332739 (C2), compared with GA (27%) and CNV only (10%).
Conclusion
There is a paucity of reports describing simultaneous CNV–GA. Clinical and genetic results may support the fact that GA and CNV fit on an age-related macular degeneration (AMD)-disease continuum and may clarify the disease processes in AMD.
Similar content being viewed by others
Introduction
Of the estimated 60 million individuals 55 and older in the United States, at least 8 million persons are affected by intermediate to advanced age-related macular degeneration (AMD).1 These people are at high risk for bilateral advanced disease, resulting in significant visual impairment.
AMD is a complex progressive disease that presents in two forms: wet (exudative) and dry (atrophic). Dry AMD can be further classified into either early changes with drusen only or late changes with extensive loss of choriocapillaris, the retinal pigment epithelium layer (RPE) and the retina, known as geographic atrophy (GA). Wet AMD, choroidal neovascularization (CNV), is characterized by the invasion of the subretinal or sub-RPE spaces with neovascular complexes that are prone to leakage. Most literature focuses on either the wet or the dry form separately. There is a paucity of reports on both forms of AMD concurrently in the same eye.
Early AMD includes soft drusen and RPE changes. When the drusen become enlarged and confluent, there is an increased risk for the progression to advanced AMD. Advanced AMD is then described as either GA or CNV.2 It is rare that they present simultaneously in the same eye. The Age-Related Eye Disease Study (AREDS) reported 14 (0.4%) of 3212 eyes, with pigmentary abnormalities at baseline, developed advanced CNV and GA by the 5-year follow up.2 In addition, the submacular surgery trial 3 reported 2.7% of 110 fellow eyes developed concomitant GA and CNV after 48 months.3
A few studies have focused on the association of GA and CNV. The Macular Photocoagulation Study (MPS) reported 5-year rates of developing CNV in an eye with GA that has a fellow eye with CNV of 45 and 49%, based on two studies.4 MPS also found significant risk factors for developing CNV in the fellow eye to be large drusen, five or more drusen greater than 63 μm, focal hyperpigmentation, and systemic hypertension.4 In addition, Sunness et al5 more recently reported that the strongest risk factor for the development of CNV in an eye with GA was the presence of CNV in the fellow eye.
Genetics and AMD
Despite the prevalence of AMD, its aetiology remains largely unknown. Recent studies have begun to further associate AMD with certain genotypic variants of complement factor H (CFH),6, 7 high temperature requirement factor HTRA1/LOC387715,6, 8, 9 complement factors 2 (C2),10, 11 complement factor 3 (C3)11, 12, 13 and B (CFB).10, 11 However, the data is still not complete, especially that related to specific subtypes of AMD. Previous studies have shown an association of GA to HTRA1/LOC387715, CFH, C2, and C3.14, 15 HTRA1 originally was related to the progression of CNV,16 but another study found HTRA1 to have a significant association with both forms of advance AMD—the wet and dry forms.17 It has also been demonstrated that CFH confers more risk to the bilaterality of GA whereas HTRA1/LOC387715 contributes more to the bilaterality of CNV.18 Another subgroup analysis showed a highly significant association with C3 and CNV in both case–control groups in the population, whereas the association with C3 and GA was only significant in one group.13 Therefore, with family history as a significant risk factor for AMD and the discovery of the relation of genetic variants to AMD, it is convincing that genetics has a strong role in the development, type, and prognosis of AMD.
The aim of this report is to study genotypes of patients presenting clinically with simultaneous GA and CNV and to compare it with genotypes of patients presenting with GA or CNV only. We wish to determine if any of the currently known genotypes is particularly associated with occurrence of simultaneous GA and CNV in patients with late AMD.
Methods
This study was approved by the Institutional Review Board of the University of California, San Diego. All subjects signed informed consent before participation in the study and before blood draw. AMD subjects were recruited at the Shiley Eye Center at the University of California, San Diego. All participants had at least two standard complete ophthalmic examinations. Exams included best-corrected visual acuity measurements and applanation tonometry for intraocular pressure measurement. ‘Baseline’ best-corrected visual acuity (BCVA) was the BCVA at the time of first diagnosis and ‘current’ BCVA was the BCVA after at least two standard ophthalmic exams. Slit lamp exams, including fundus biomicroscopy using a 90-dioptre lens, were performed. Binocular indirect ophthalmoscopy was performed using a 20-dioptre lens. In addition, fundus photography, fluorescein angiography (FA) and optical coherence tomography (OCT) were completed.
Study procedures
Stereo fundus photography and FA were completed on all patients after adequate dilation. A pair of stereoscopic color fundus photographs (50 degrees) were taken, centred on the fovea using a Topcon fundus camera (Topcon TRV-50VT, Topcon Optical Company, Tokyo, Japan). A fluorescein angiogram was obtained in a standard fashion using a Heidelberg Retina Tomograph 2, (Heidelberg Engineering, Heidelberg, Germany) OCT images were obtained using a Topcon 3D OCT-1000 (Topcon Optical Company) by a trained ophthalmic technician. The scanning protocol was the macular thickness and raster scan sequence that creates a retinal map algorithm. For this study, mean foveal thickness value was used as the OCT measurement of foveal thickness.
Patients with concomitant CNV–GA were chosen based on clinical exam and diagnostic imaging, including fundus photos, OCT and FA. Fundus exam showed a combination of subretinal or sub-RPE fluid or haemorrhage, as well as a well-demarcated area of decreased retinal thickness and change in retinal colour, also known as GA. All GA lesions were at least 500 square microns in diameter as measured by HRA2 instrument. In addition, FA confirmed vascular leakage and OCT imaging revealed the presence of fluid within the retina. Seven patients were affected in the right eye only, four patients in the left eye only and nine patients in both eyes. In patients affected in both eyes, each eye was evaluated.
Genotyping
Genomic DNA samples were extracted from peripheral blood leucocytes according to established protocols. Six single-nucleotide polymorphisms (SNPs), in four genes associated with AMD, were selected according to the literature. They were rs2274700 and rs1061170 in CFH,6, 7 rs10490924 and rs1120638 in HTRA1/LOC387715,6, 8, 9 rs2230199 in C314, 15 and rs9332739 in C2.14, 15 These specific SNPs were selected because the most convincing evidence for a genetic contribution to AMD was the identification of major disease susceptibility alleles on chromosome 1q32, which includes the gene for CFH and HTRA1/LOC387715. The literature has also shown C3 and C2 to be candidate genes.14, 15
All SNPs were genotyped using the SNaPshot method according to the manufacturer’s recommendations. In brief, a SNP was amplified by PCR, the PCR product purified by Exo I and shrimp alkaline phosphatase (SAP) (New England Biolabs, Ipswich, MA, USA). The purified PCR product and the SNaPshot primer were then used to perform a single-base pair extension with the SNaPshot multiplex mix (Applied Biosystems Inc., Foster City, CA, USA). After an additional purification step using SAP, the product was run and analysed on an ABI 3130xI genetic analyser (Applied Biosystems Inc.) and genotyping results were obtained directly.
Statistical analysis
In order to compare patients with CNV–GA to both the control groups—GA only and CNV only patients—we conducted chi-squared tests. We compared the genotypic frequencies and the allelic frequencies of the CNV only group to patients with CNV–GA for each SNP analysed, focusing on either the at-risk genotype or allele for genes known to potentially increase the risk of AMD or the protective genotype or allele known to potentially protect against AMD. Then, we compared the genotypic frequencies and allelic frequencies between the GA only group and the CNV–GA group for each SNP analysed. For example, if we were comparing two groups for genotypic frequency at the HTRA1 SNP rs10490924 then we would be comparing the frequency of the TT genotype between the two groups. If we were comparing two groups for allelic frequency at the HTRA1 SNP rs10490424 then we would be comparing the frequency of the T allele between the two groups. For the remainder of the at-risk SNPs analysed, we compared the AA genotype and A allele frequency for rs11200638, the CC genotype and C allele for rs2774700, the CC genotype and C allele for rs1061170, and the GG genotype and G allele for rs2230199. For rs9332739, we compared the protective CC genotype and C allele.
Results
A total of 20 patients met study criteria for diagnosis of simultaneous CNV–GA. A hundred and fifty-four CNV only and 154 GA only age-matched patients were recruited and compared with the patients with combined CNV–GA. Of the patients with combined CNV–GA, average age was 75 years with 13 females and 7 males. All 20 patients identified themselves as Caucasian–European. Nine patients were previous smokers for at least 10 years and one patient is a current smoker (Tables 1a and b). Of the 154 age-matched GA patients, the average age was 82, consisting of 60 males and 94 females. There were two patients identified as Hispanic, the remainder were all Caucasian–European. Thirteen patients stated they were current smokers with 43 past smokers. (See Supplementary Table S1 for details.) Of the 154 age-matched CNV patients, the average age was 81, with 46 males and 108 females. There were two Hispanic patients, one Asian patient and the remainders were Caucasian. Twelve patients were current smokers and 84 were past smokers.
Average best-corrected visual acuity (BCVA) measurements for baseline was 20/185 and for current visual acuity (VA) was 20/230 for eyes in patients with CNV–GA (Table 1a). The baseline VA was taken at the initial ophthalmic visit and the current VA is the most recent measurement taken for each patient. Of the twenty patients with CNV–GA, eight patients had at least one eye maintain the same BCVA overtime and three patients had at least one eye improve by at least one line. Eight patients experienced worsening VA in at least one eye. Four patients did not have a baseline VA due to prior treatment at another facility.
In patients with CNV–GA, GA progressed slowly and often preceded CNV. CNV presented as subretinal haemorrhage (Figure 1a), fluid without haemorrhage, or drop in VA with questionable presence of fluid. When CNV developed simultaneously, there was often an abrupt drop in VA, with average current VA of 20/200. CNV presented in the centre of the GA, at periphery of the GA, or at a distance from the GA. Imaging from a representative patient with CNV–GA is displayed in Figures 1 and 2.
(a) Colour fundus photograph demonstrating simultaneous GA and CNV. A large area of GA with discrete borders is noted temporal to the fovea with an area of subretinal haemorrhage on the inferior border of the GA. (b) OCT imaging of the retina in the same eye reveals areas of subretinal fluid and intraretinal cysts.
(a) Early-phase flourescein angiogram again in the same eye demonstrating transmission hyperfluorescence in the area of GA. (b) Late-phase fluorescein angiogram showing some fading of the choroidal fluorescence and with staining hyperfluorescence of the region of GA. In addition, mild leakage of fluorescein dye is noted in the infero-temporal region of the area of GA. (c) Early-phase Idocyanine green (ICG) angiogram showing underlying choroidal vasculature. (d) Late-phase ICG angiogram confirming area of leakage.
In comparing GA–CNV group to GA only and CNV only group, respectively, no significant difference in either genotypic or allelic frequency of each gene was found (Table 2). However, the patients with CNV–GA had a higher frequency of at-risk alleles at both SNPs within the HTRA1 gene—rs10490924 (52.5%) and rs11200638 (52.6%) when comparing to GA only patients (40.2, 46.2%) or CNV only patients (42.9, 41.9%) (Table 2a). Interestingly, the risk allele frequencies of SNPs in the other major gene of AMD- CFH- rs2774700 (70%) and rs1061170 (50%) were lower than those in GA only patients (81, 58.2%) and CNV only patients (78.2, 55.8%) (Table 2a). Although none of the differences showed statistical significance (Table 2a), there is a potential trend in this cohort.
In addition, CNV–GA patients was also found to have no protective alleles at the C2 gene SNP rs9332739, compared with 27% in the GA only patients and 10% in the CNV only patients (Table 2a). However, this relationship did not show a statistically significant association between either CNV only and CNV+GA or GA only and CNV+GA patients with P-values of 0.289 and 0.531, respectively (Table 2a).
Discussion
CNV and GA are most commonly discussed as if they are separate diseases, resulting from different genetic at-risk markers and biochemical pathways, and that they are not one disease on a continuum. If this statement were true, then it would be reasonable to assume that if a patient is diagnosed with both GA and CNV simultaneously in one eye, that they would have a higher proportion of at-risk alleles. In addition, they would have higher incidence of those alleles that have been associated with both GA and CNV.
Just as the presentation of GA and CNV together would most likely be characterized as a more advanced stage of disease, the bilaterality of disease corresponds to a more severe stage of disease or a sign of progression. Previous studies have found that at-risk alleles for AMD are more common in bilaterally affected individuals with AMD, compared with the unilaterally affected group.18
However, in our analysis of patients with both CNV and GA simultaneously, we have found no statistical significance between GA only and CNV only and patients with concomitant CNV and GA. Therefore, it can be assumed that, based on these known genes analysed, that GA and CNV may fit on a continuum, in that patients with GA will at some point potentially develop CNV and that they are not separate diseases after all.
Previous studies have shown certain risk genes with a higher association with one phenotype compared with the other. For example, DeWan et al19 reported HTRA1/LOC387715 as a risk gene for CNV, implicating certain polymorphisms within this gene to being responsible for this specific phenotype of AMD. Yet other groups have found no difference between GA and CNV,18 which would further support the notion that they are not two separate diseases. In addition, another study found C3 rs2230199 predisposes to GA more than CNV,18 but further investigation is needed to explain this phenomenon. Another study found that among patients with CNV in one eye and GA in the fellow eye, the cumulative incidence of CNV in the eye with GA is 30–50% at approximately 5-years’ follow up.5 Histopathological studies have also indicated that CNV is present in approximately one-third of cases with GA.20, 21 These studies further confirm the likelihood that these two phenotypes may be representing a single disease at individual stages in the progression of the disease.
This is significant because until CNV develops in eye with GA, these two forms of advanced AMD have generally been treated as different diseases and they have also generally been managed using separate treatment regimens. Patients with wet AMD are treated with intravitreal injections of anti-VEGF medications or laser and patients with dry AMD are commonly placed on AREDS vitamin formula and observed for signs of progression. However, if these diseases do lie on a continuum, then established or future medications could potentially benefit both forms of advanced AMD. For example, fenretinide, a once daily oral medication believed to decrease a toxic byproduct of retinol in the retina, has been shown to reduce the incidence of emerging CNV, in patients with GA and may slow the progression of dry to wet AMD.22
More information regarding the pathogenesis of AMD and the biochemical pathways that lead to distinct phenotypes need to be further characterized. Implicit in our study and several others is the idea that AMD can be characterized as a linear progression from one stage of the disease to the next, supported by the observation that GA usually develops before CNV. This idea still may be incorrect, and patients with simultaneous CNV and GA may have two separate biochemical pathways operating at the same time, but our study may support the opposite. Our study has only shown this potential trend in a small group. With improved characterization of the disease, we will be better equipped to treat the disease at its separate stages of progression.

References
Age-Related Eye Disease Study Group Potential public health impact of age-related eye disease study results. Arch Ophthalmol 2003; 121: 1621–1624.
Age-Related Eye Disease Study Group The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005; 123 (11): 1484–1498.
Schatz H, McDonald HR . Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology 1989; 96: 1541–1551.
Macular Photocoagulation Study Group Risk factors for neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol 1997; 115 (6): 741–747.
Sunness JS, Gonzales-Baron J, Bressler NM, Hawkins B, Applegate CA . The development of choroidal neovascularization in eyes with the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999; 106: 910–919.
Swaroop A, Chew EY, Rickman CB, Abecasts GR . Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet 2009; 10: 19–43.
Thakkinatian A, Han P, McEvoy M, Smith W, Hoh J, Magnusson K et al. Systematic review and meta-analysis of the association between complement factor HY402H polymorphisms and age-related macular degeneration. Hum Mol Genet 2006; 15: 2784–2790.
Ross RJ, Verma V, Rosenberg KI, Chan CC, Tuo J . Genetic markers and biomarkers for age-related macular degeneration. Expert Rev Ophthalmol 2007; 2 (3): 443–457.
Tang NP, Zhou B, Wang B, Yu RB . HTRA1 promotor polymorphism and risk of age-related macular degeneration: a meta-analysis. Am Epidemiol 19: 740–745.
Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet 2006; 38: 458–462.
Francis PJ, Hamon Sc, Ott J, Weleber RG, Klein ML . Polymorphisms in C2, CFB and C3 are associated with progression to advance age related macular degeneration associated with visual loss. J Med Genet 46: 300–307.
Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM . Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet 2007; 39: 1200–1201.
Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med 2007; 357: 553–561.
Klein ML, Ferris FL, Francis PJ, Linblad AS, Chew EY, Hamon SC et al. Progression of geographic atrophy and genotype in age-related macular degeneration. Ophthalmology 2010; 117 (8): 1554–1559.
Scholl HP, Fleckenstein M, Fritsche LG, Schmitz-Valckenberg S, Göbel A, Adrion C et al. CFH, C3 and ARMS2 are significant risk loci for susceptibility but not for disease progression of geographic atrophy due to AMD. PLoS One 2009; 4 (10): e7418.
Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML . Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA 2007; 267 (16): 1793–1800.
Cameron DJ, Yang Z, Gibbs D, Chen H, Kaminoh Y, Jorgesen A et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle 2007; 6 (9): 1122–1125.
Chen Y, Zeng J, Zhao C, Wang K, Trood E, Buehler J et al. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch Ophtalmol 2011; 129 (3): 344–351.
Dewan A, Lui M, Hartman S, Zhang SS, Liu DT, Zhao C et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006; 314 (5801): 989–992.
Sarks SH . Aging and degeneration in the macular region: a clinicopathologic study. Br J Ophthalmol 1976; 60: 324–341.
Green WR, Enger C . Age-related macular degeneration histopathologic studies. Ophthalmology 1993; 100: 1519–1535.
Mata NL, Tsivkovaskaia N, Bui TV . Revision therapeutics. Fenretinide reduces the incidence of choroidal neovascularization in patients with geographic atrophy. ARVO Annual Meeting, 2 May, 2011. Session 258. Poster 1652.
Acknowledgements
This study was approved by the Institutional Review Board of the University of California, San Diego. All subjects signed informed consent before participation in the study. We thank Drs Yuhong Chen and Peter Shaw for their help with genotyping. This work was supported by NEI/NIH grants, research to prevent blindness, San Diego Clinical and Translational Research Institute 1TL1RR031979-01 and the VA Merit Award.
Author contributions
Design of study (SG, IK, KZ); conduct of study (SG, GH); data collection (SG, JL, CL, HF, WF, IK); Analysis and interpretation of data (SG, HD, JL, IK, KZ); Writing and preparation of manuscript (SG, IK, KZ).
Disclaimer
The principal investigator, Dr Kang Zhang, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Grob, S., Luo, J., Hughes, G. et al. Genetic analysis of simultaneous geographic atrophy and choroidal neovascularization. Eye 26, 1106–1113 (2012). https://doi.org/10.1038/eye.2012.107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.107