Abstract
Purpose
To analyze Humphrey visual field (HVF) findings in hydroxychloroquine (HCQ) retinal toxicity.
Methods
HVF tests were interpreted retrospectively in this observational case series of 15 patients with HCQ toxicity. Patients seen at Lahey Clinic were identified by diagnosis coding search. Patients with age-related macular degeneration or glaucoma with visual field loss were excluded. HVFs done before the diagnosis were analyzed to see if earlier diagnosis could have been possible.
Results
A total of 66 HVFs were reviewed and categorized. Some abnormalities were subtle. Paracentral defects were seen on 10-2 tests whereas 24-2 tests, due to their compressed scale, showed central changes. The abnormalities were often more obvious on pattern deviation rather than the gray scale. Of those patients with prior HVFs available for review, 50% showed HVF abnormalities typical of HCQ toxicity present several months or years before diagnosis. HVF changes preceded fundus changes in nine patients.
Conclusion
HVF abnormalities indicating HCQ toxicity vary depending on the specific HVF test performed. Clinicians need to be aware of the subtle nature of HVF changes in early toxicity.
Similar content being viewed by others
Introduction
Misinterpretation of Humphrey visual field (HVF) findings in patients utilizing hydroxychloroquine (HCQ) can lead to delay in diagnosis of retinal toxicity. Early retinopathy has been defined as an acquired, persistent paracentral scotoma visualized on automated visual field testing without any observable fundus changes.1 Reports have shown that the visual loss from HCQ toxicity is less likely to be reversible once fundus changes are present.1,2,3 Although newer testing modalities, such as spectral domain-optical coherence tomography, fundus autofluorescenece, and multifocal electroretinogram, are now recommended as part of screening when available,2 automated perimetry remains the most readily available screening test in the majority of practices. Additionally, eye care providers are familiar with interpretation of HVF for glaucoma and other conditions. However, the HVF changes that occur in HCQ toxicity are not those seen in glaucoma. Although there have been descriptions in the literature of HVF findings in HCQ toxicity,1,4,5,6 we find that there is confusion in ophthalmology and optometry communities about this topic. HVF findings that may be diagnostic of early HCQ toxicity need to be well understood if clinicians are to be successful at making the diagnosis, particularly when there are no fundus changes to raise suspicion.
The purpose of this study is to review the visual field findings of patients who have been previously diagnosed with HCQ retinal toxicity and to present a summary of HVF patterns seen with this condition, including cases of early toxicity. A secondary goal is to determine if changes could have been recognized earlier in retrospective review of visual field testing done before the time of diagnosis. We will attempt to determine factors that contributed to delay of diagnosis.
Materials and methods
The institutional review board at Lahey Clinic approved this retrospective study. Charts were reviewed for patients diagnosed with HCQ toxicity at Lahey Clinic. Patients were identified via a diagnosis coding data search for HCQ toxicity. Medical records were requested from outside ophthalmology offices for patients referred to Lahey for evaluation and treatment.
In all, 16 patients were diagnosed with HCQ toxicity between the years 2001 and 2010. One patient was excluded from the study as she did not have any HVF tests for review within 2 years of diagnosis. This patient was diagnosed based on fundus exam. In the remaining 15 patients, diagnosis was based upon reproducible paracentral or central scotoma on automated visual field testing or a single abnormal HVF test combined with typical fundus changes, as per diagnostic criteria of Bernstein.7 Patients with significant age-related macular degeneration or severe glaucoma were excluded from the study. Patients with mild glaucoma or drusen without atrophy were not excluded from this study but the co-morbid conditions are noted in the analysis.
Information collected included the type(s) of HVF performed, the date of diagnosis, or date that HCQ was discontinued, and the pattern or defect on HVF. Dosages, length of treatment, and results of other screening tests including fundus exam were noted. In this retrospective study, we quote dosages based on actual, self-reported weight recorded in the chart.
Results
In all, 66 HVFs of 15 patients with HCQ toxicity were reviewed. Different HVF strategies had been used for toxicity screening in this cohort of patients referred from a variety of providers. In all, 52 (79%) of the tests were 10-2 test strategies: with slightly over half of these being red target as compared with white target tests. The remaining 14 HVFs were 24-2 and 30-2 tests.
An average of 4.4 HVFs per patient were available for review with a range of 1–16 HVFs per patient. Nine patients showed a significant period of time where there was no HVF performed either because the patient was lost to follow-up for several years (five patients) or the provider did not order the HVF test as part of screening (four patients). In another three patients, we did not have access to any additional HVFs that may have been performed before referral.
Six patients in this series had pigmentary changes noted at the time of diagnosis. Another patient had drusen but normal IVFA at a time when they showed abnormal HVF typical for HCQ toxicity. The remaining eight patients had a normal fundus exam at the time of diagnosis, and therefore would be considered early toxicity by our criteria. Table 1 shows dosages, length of treatment, fundus findings at time of diagnosis, and results of ancillary testing for each patient. All dosages are reported based on the patient reported weight (rather than lean body weight). All patients were on an actual dose of 400 mg/day, except for patients #1 and 11 who started at that dose and then decreased to 200 mg daily as detailed in the table.
In all, 11 patients had been using the medication for 15 years or longer. None of our patients had renal disease, which is a risk factor for HCQ toxicity.
Visual field findings
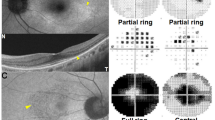
We reviewed 25 10-2 white tests in 10 of our patients with toxicity. Figure 1 shows a dense, well-defined paracentral ring scotoma in a patient who already had pigmentary fundus changes at the time of referral (patient 3). The remainder of the patients tested with 10-2 white showed more subtle findings. In half of the patients who had 10-2 white tests, the HVF gray scale showed bilateral partial ring defects sparing the central 2 degrees. A complete ring (also sparing the central 2 degrees) was present in the remaining 5 patients who were evaluated by 10-2 white tests. There was no correlation with complete vs partial ring scotoma and presence or absence of fundus changes. Almost all the 10-2 white HVFs showed a defect predictably within the same area: 2–6 degrees from fixation on gray scale, sparing the central 2 degrees. However, two patients showed scattered patches of relative scotoma extending out to 10 degrees, but still sparing the central 2 degrees. Figures 2, 3, and 6 (bottom) show 10-2 white tests from five different patients demonstrating slightly different variations of a paracentral ring defect. In subtle cases, the defect was more obvious when looking at the pattern deviation. In three patients (four tests) the 10-2 white test interpreted as normal by the eye care provider showed a defect suggestive of HCQ toxicity on our retrospective review.
Eight patients had a total of 27 10-2 red tests. Most of the 10-2 red tests showed a complete ring defect bilaterally with a darker gray scale and a wider area affected as compared with the 10-2 white tests (Figure 4). The central sparing was smaller in size and occasionally obliterated. In one of the patients diagnosed by 10-2 red test (patient #10), instead of a ring defect the HVF showed bilateral paracentral scotomas that varied significantly with each test but demonstrated progression over time to a partial ring. In three patients who had 10-2 red tests there were five tests that were read as normal initially but were suggestive of HCQ toxicity on retrospective review.
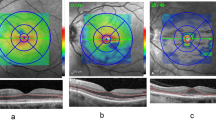
In the 24-2 and 30-2 testing strategies, because the central area is compressed, one cannot distinguish the central 2-degree field sparing seen on 10-2 tests. Therefore, the HVF pattern on the 24-2 and 30-2 tests showed a small central defect rather than a paracentral ring. Figures 5, 6 (top), and Figure 7 are examples of 24-2 testing in three different patients. We reviewed twelve 24-2 tests and two 30-2 tests in six patients. The central 4 points around fixation are where the scotomas from HCQ toxicity occur on these larger field tests. Tests were felt to be suggestive of toxicity even if there was only one central point scotoma bilaterally of P<1% probability (represented by a dark gray box on statpac, Zeiss Humphrey, Dublin, CA, USA). It should be noted that a mild, relative defect is seen more easily on deviation plots rather than gray scale. Seven of the twelve 24-2 tests and both of the 30-2 tests reviewed were misinterpreted by the referring eye care provider according to our retrospective review.
Delay of diagnosis in a patient with HCQ Toxicity. This series in a single patient shows a delay of diagnosis. Top: The 24-2 from 4/11/03 shows a central defect consistent with HCQ toxicity. Although it is subtle on the gray scale, the abnormality is evident on the pattern deviation. The test was reported as normal. Middle: A 30-2 done 6/19/03 shows a similar result, but again was read as normal. Bottom: After the patient was lost to follow-up for almost 3 years, the diagnosis was made on 3/2/06 with this 10-2 white test. The fundus remained normal.
Another example of delayed diagnosis in HCQ toxicity. Top: pattern deviation shows a defect that is difficult to see on gray scale in this 24-2 test from 8/23/05. Middle: the patient was lost to follow-up for 3½ years. The fundus was still normal on 2/21/08. The abnormality is now slightly more evident on gray scale, but was interpreted as normal. Bottom: on 3/10/09 the patient had pigmentary changes and a diagnosis of toxicity was made.
Delay of diagnosis
We were able to review HVF scans before diagnosis in 10 patients. Retrospective review showed evidence of HVF abnormality before the diagnosis of HCQ toxicity in five (50%) of those patients who had prior HVF available for review (Table 2). These five patients had 24 total HVF tests reviewed with 14 tests showing findings missed by the clinician. The delay of diagnosis ranged from 11 months to 5½ years. Figures 6 and 7 show examples of patients who could have been diagnosed earlier based on HVF test results.
The reasons for missing the HVF abnormality varied. The HVF strategy most commonly misinterpreted in this study was the larger visual field tests (24-2 and 30-2), accounting for 9 out of 14 total that were incorrectly interpreted. In one patient, the 10-2 test print out in the medical record did not include deviation plots, which in retrospect could have made the paracentral scotoma more readily apparent to the examiner. In one patient the type of HVF strategy used for screening varied several times over a 6-year period of follow-up. Therefore the comparative value of watching for progression over time on a particular test was lacking. Two of our patients had concurrent diagnoses such as drusen (patient #9) and glaucoma (patient #13), which made the interpretation of the HVF difficult according to the recorded evaluation at the time. Lastly, four of these five patients with HVF misinterpretations were also lost to follow-up for 2 years or more between the time when an abnormal HVF test is documented via retrospective review and the time the diagnosis was made by the practitioner. This fact contributed to the length of time the diagnosis was delayed.
Discussion
The patterns seen on HVF in this cohort of patients with HCQ toxicity depended on the type of test used. The 10-2 white test was felt by the authors to be the most reproducible and easiest to interpret. Although visual field defects in HCQ toxicity can be highly variable in terms of the depth of defect, all our patients showed a similar pattern on 10-2 white, with the same areas affected. However, one needs to look carefully for subtle decreases in threshold in the area at risk: 2–6 degrees from center. On 10-2 white HVF even mildly decreased thresholds (4–8 dB) could be recognizable as positive when there was a contiguous pattern of a paracentral ring or partial ring (Figure 2). One also needs to be certain to review the deviation plots,2 as this may make subtle defects more obvious. Previous literature has reported that the superior visual field is most likely to be affected first.3 We noted the superior field to be somewhat more affected than inferior field in 7/15 of our patients. The difference was noted in patients with subtle HVF findings as opposed to those with more dense HVF abnormalities. One of the 15 patients showed the inferior field to be slightly more affected than the superior field. In the remaining seven patients, the superior and inferior fields were equally affected.
When evaluating the 24-2 HVF in HCQ screening, one must pay particular attention to the 4 points surrounding fixation on the plot. Unlike in glaucoma, a single point scotoma may be very significant in HCQ toxicity. A lack of understanding of what to expect on 24-2 or 30-2 tests in HCQ toxicity may have contributed to delay of diagnosis in four patients (nine tests) in this cohort. As with 10-2 white tests, we found it was visually easier to detect a subtle abnormality on the pattern deviation than on the gray scale plot on 24-2 tests.
The 10-2 red test has been recommended in the past as the testing strategy of choice for HCQ toxicity due to its sensitivity.1,5 Easterbrook1 reported the 10-2 red test to be 91% sensitive for detection of HCQ toxicity. However, he also reported a low specificity of 57% for this test. For the 10-2 white test he found a lower sensitivity of 78% for detection of HCQ toxicity but better specificity of 84% with the 10-2 white test (Figure 8).
Comparison of red target vs white target 10-2 tests. The two tests shown are from a single patient (#13) done 1 day apart. The red target test was felt to be a false positive as it was so dense. Note that the very central 3–4 degrees are relatively less affected, which is typical of HCQ toxicity. The white target test, read as normal, shows decreased threshold in the peri-central region, suggestive of toxicity. Because of misinterpretation, this was a false negative test. In retrospect, both the red target and white target tests should have been seen as supporting the diagnosis of toxicity.
Although our numbers in this series are too small to give any reliable sensitivity or specificity data, the authors feel more comfortable with the 10-2 white test when screening for HCQ toxicity due to the predictability of the defect seen in this cohort. The 10-2 white strategy is also recommended in a recent American Academy of Ophthalmology publication regarding HCQ screening recommendations.2 The exception to our use of 10-2 white strategy would be for a patient with concurrent glaucoma. When there are other clinical reasons to perform 24-2 or 30-2 tests, the clinician can use that test for HCQ screening but must be aware of how HCQ toxicity manifests on the larger-scale HVF testing strategies as elucidated above. The 10-2 white testing can then be performed for confirmation. However, it is not recommended to routinely switch between protocols. We believe the lack of consistency of HVF screening strategy used may have contributed to a significant delay of diagnosis in one of the patients in this cohort.
Although HCQ toxicity is indeed uncommon when the appropriate dose is used and when there are no other risk factors (obesity, renal disease, or excessive duration of treatment), toxicity does occur. Recent studies indicate the prevalence may be higher than previously believed,2,6,8 ∼1% after 5–7 years of use and increasing to 2% for 10–15 years of use according to Wolfe et al.8 It is important that eye care providers are familiar with the patterns on HVF that indicate toxicity: partial or complete ring defect between 2–6 degrees with central sparing on 10-2 white tests; central scotoma affecting one or more of the 4 points around fixation on 24-2 testing (more evident on deviation plots); or more diffuse central or paracentral defect on 10-2 red visual field testing.

References
Easterbrook E . Ocular effects and safety of antimalarial agents. Am J Med 1988; 85: 23–29.
Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF . Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011; 118: 415–422.
Hart WM, Burde RM, Johnston GP, Drews RC . Static perimetry in chloroquine retinopathy: perifoveal patterns of visual field depression. Arch Ophthalmol 1984; 102: 377–380.
Elder M, Rahman AM, McLay J . Early paracentral visual field loss in patients taking hydroxychloroquine. Arch Ophthal 2006; 24: 1729–1733.
Johnson MW, Vine AK . Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Opthal 1987; 104: 139–144.
Michaelides M, Stover NB, Francis PJ, Weleber RG . Retinal toxicity associated with hydroxychloroquine and chloroquine. Arch Ophthal 2011; 129: 30–39.
Bernstein HN . Ocular safety of hydroxychloroquine. Ann Ophthalmol 1991; 23: 292–296.
Wolfe F, Marmor MF . Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care and Res 2010; 62: 775–784.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part as a poster at AAO Annual Meeting, San Francisco, CA, USA; October 2009
Rights and permissions
About this article
Cite this article
Anderson, C., Blaha, G. & Marx, J. Humphrey visual field findings in hydroxychloroquine toxicity. Eye 25, 1535–1545 (2011). https://doi.org/10.1038/eye.2011.245
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.245
Keywords
This article is cited by
-
Visual field examinations using different strategies in Asian patients taking hydroxychloroquine
Scientific Reports (2022)
-
Clock-hour topography and extent of outer retinal damage in hydroxychloroquine retinopathy
Scientific Reports (2022)
-
An objective method of diagnosing hydroxychloroquine maculopathy
Eye (2021)
-
The effect of oral acetazolamide on cystoid macular edema in hydroxychloroquine retinopathy: a case report
BMC Ophthalmology (2017)
-
A Critical Review of the Effects of Hydroxychloroquine and Chloroquine on the Eye
Clinical Reviews in Allergy & Immunology (2015)