Abstract
Neuro-osteological interactions have an important role in the regulation of bone metabolism and regeneration. Neuropeptides combined with porous biphasic calcium phosphates (BCP) using protein adsorption may contribute to the acceleration of bone formation. In the present study, we investigated the effect of BCP combined with nerve growth factor (NGF) on the growth of osteoblasts in vitro and the combinational therapeutic effect on the repair of calvarial defects in vivo. NGF was separated and purified from Chinese cobra venom using a simplified three-step chromatography method. BCP combined with NGF exerted a potent effect on osteoblast differentiation, as evidenced by enhanced cell proliferation, increased ALP activity and the up-regulated expression of osteogenesis-related genes and proteins. Further, combinational therapy with BCP and NGF improved calvarial regeneration, which was superior to treatment with therapy alone, as observed using imageological and morphological examination and histological and immunohistochemical staining. The results confirmed the effect of neuro-osteological interactions through combinatorial treatment with NGF and BCP to promote osteogenesis and bone formation, which may provide an effective and economical strategy for clinical application.
Similar content being viewed by others
Introduction
Calcium phosphate (Ca-P) ceramics have been widely used as bone substitutes in the restoration of calvarial defects and lateral, vertical bone augmentation models.1, 2, 3, 4 Among various Ca-P ceramics, porous biphasic calcium phosphate ceramics (BCP) have attracted much attention in the reconstruction of large and complex bone defects because of their excellent osteoconduction and proper degradation.5, 6, 7 However, a rather long duration of the bone consolidation phase is typically required after therapy using BCP, which may elicit unexpected complications and inconvenience care clinics. It is important to identify an effective method to accelerate bone formation and reduce the duration of this therapy.
It has been proposed that bone metabolism is regulated by neuro-osteological interactions.8, 9, 10 Multiple studies have revealed that a number of neuropeptides secreted by the skeletal nerve fibers exert a robust effect on osseous formation and bone resorption.10, 11 Among the numerous neurotrophins, nerve growth factor (NGF) is frequently used in bone defect therapies.12, 13, 14 NGF exists in many animals and can be extracted from submaxillary salivary glands, bovine seminal plasma, snake venom and so on15, 16, 17, 18, 19, 20, 21 Among these sources, snake venom-derived NGF exhibits better bioactivity and stability than the venom from other sources.15, 16, 17 NGF stimulates bone formation around the induced regenerating axons and improves bone fracture healing.22, 23 The injection of NGF is a useful method to stimulate bone formation14, 24, 25 NGF can also be delivered using various scaffolds, including agarose hydrogel, copolymers (PPy-NSE), poly-lactic-co-glycolic-acid (PLGA) and so on.26, 27, 28, 29, 30, 31, 32 However, most of the carriers for NGF are hydrogels or porous polymers that can hardly achieve the functional reconstruction of large-scale defects. A combination of NGF with Ca-P ceramics, such as BCP, may hold promise in the therapy of extensively damaged bone.
A composite system comprising inorganic phases and organic substrates is difficult to prepare. Although proteins can be immobilized onto the surfaces of ceramics by chemical modifications to form covalent bonds, protein properties, including the structure, conformation or aggregation, may be altered. Alternatively, ceramics can be used to absorb proteins. Emerging evidence indicates that Ca-P ceramics have protein adsorption capacity, which may underlie the osteoinduction observed after implantation in vivo. In addition, Ca-P ceramics have also been exploited for protein separation and protein anchorage.33 In particular, BCP is promising in the adsorption of proteins, as these molecules easily form microporous structures and exhibit an affinity for proteins.34, 35, 36 Proteins, such as fibronectin, bone sialoprotein and transforming growth factor, are selectively adsorbed onto the surface of Ca-P ceramics.35, 37, 38 This feature may be useful for combinational therapy using BCP and neuropeptides, such as NGF, for bone defects.
In the present study, we investigated the role of BCP combined with NGF from Guangxi cobra venom on the growth of osteoblasts in vitro and the repair of calvarial defects in vivo. To separate and purify the Chinese cobra venom, we improved upon a simplified three-step chromatography method described in a previous study.39, 40 NGF was retained by BCP using protein absorption. Thus, the aim of the present study was to employ neuro-osteological interactions to shorten the duration of the bone consolidation phase.
Materials and methods
Purification of NGF
As shown in Figure 1A, crude Guangxi cobra venom was sequentially separated on Sephadex G-50, CM Sepharose FF and Superdex 75 PG chromatography columns. Briefly, 2 g lyophilized cobra venom powder was thoroughly dissolved in 10 ml of 1% acetic acid (HAC, Sigma-Aldrich, St Louis, MO, USA) at 4 °C. After centrifugation at 12,000 r.p.m. for 10 min at 4 °C, the supernatant was collected, passed through Sephadex G-50 (GE Healthcare Bio-Sciences China Ltd, Beijing, China) at the rate of 1 ml min−1, and eluted with 1% acetic acid. A 10-ml fraction containing NGF activity was obtained and loaded onto CM Sepharose FF (GE Healthcare Bio-Sciences China) after overnight dialysis and gradient elution with 0.02 mol l−1 sodium acetate-acetic acid (NaAC-HAC, pH 5.8, Sigma-Aldrich) ranging from 0 to 1 mol l−1 sodium chloride (NaCl, Sigma-Aldrich). The fraction with NGF activity was collected and slowly separated on Superdex 75 pg (GE Healthcare Bio-Sciences China) at 0.8 ml min−1. After elution with 1% HAC, the fraction with NGF activity was collected, dialyzed for desalination and lyophilized. Finally, the purified NGF was dissolved in normal saline at a concentration of 0.1 mg ml−1 as a stock solution and stored at −20 °C.
Purification and bioactivity assay of nerve growth factor (NGF) from crude Guangxi cobra venom. (A) Purification of NGF. (B) Chromatogram of crude venom on Sephadex G-50. Symbols 1, 2, 3 and 4 separately denote the isolated chromatographic peaks. (C) Chromatogram of crude venom on a CM Sepharose FF column. Symbols 1, 2, 3, 4, 5, 6, 7 and 8 separately denote the isolated chromatographic peaks. (D) Chromatogram of the NGF fraction on a Superdex 75 PG column. Symbols 1 and 2 separately denote the isolated chromatographic peaks. (E) Electropherogram of NGF and model proteins on SDS-PAGE. (F (a,b)) PC12 cells cultured without (a) and with (b) 2 μg ml−1 NGF for 24 h (Scale bar=50 μm).
Molecular weight assay of NGF
Lyophilized powder was completely dissolved in 1% HAC, and the molecular mass was determined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, Sigma-Aldrich) SDS. Subsequently, the gel was fixed using 12% trifluoroacetic acid (Sigma-Aldrich) and stained with Coomassie Blue R-250 (Shanghai Solarbio Science and Technology, Shanghai, China).
Bioactivity assay of NGF
PC12 cells (purchased from Institute of Cell Biology, Chinese Academy of Science, Shanghai, China) were cultured in alpha-modified Eagle’s medium (α-MEM, Gibco BRL, Gaithersburg, MD, USA) containing 10% (v/v) fetal bovine serum (FBS, Gibco BRL) and 1% (v/v) antibiotics (penicillin 100 U ml−1, streptomycin 100 U ml−1, Beijing Solarbio Science and Technology, Beijing, China) with or without 2 μg ml−1 NGF. NGF bioactivity was verified based on morphological changes of PC12 cells after 24 h.
In vitro experiment
Primary osteoblasts were harvested from the bilateral parietal bone of newborn Sprague–Dawley (SD) rats (Animal Resources Center of Guangxi Medical University, Nanning, Guangxi, China) using enzymatic digestion. After being stripped clearly with sterile gauze and digested with 0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) (Beijing Solarbio Science and Technology), the bilateral parietal bones were cut into pieces and subsequently digested with 1 mg ml−1 collagenase type I (Gibco BRL) for 3 h. After centrifugation, the isolated cells were resuspended and subsequently cultured at 37 °C in a 5% CO2 incubator (Thermo Scientific TM Forma Series II Water-Jacketed, Santa Ana, CA, USA), and the medium was changed every other day.
Preliminary screening and cytotoxicity assay
Preliminary screening and cytotoxicity analysis were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) method. For preliminary screening, the cells were cultured with NGF ranging from 0.625 to 250 μg ml−1 in monolayer or BCP-based three-dimensional (3D) cultures. BCP (HA/β-TCP=60/40, diameter=12 mm, thickness=2 mm, porosity=75%±5%) blocks were purchased from National Engineering Research Center in Biomaterials of Sichuan University of China. After preliminary screening, optimal concentrations of NGF were selected for further cytotoxicity analysis using an MTT assay in both 2D and 3D culture systems after 2, 5 and 8 days. Briefly, MTT was added to each well at a final concentration of 0.5 mg ml−1. After incubation for 4 h, 1 ml dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added for formazan-crystal solubilization. The absorbance was measured at 570 nm using a microplate reader (Thermo Scientific Multiskan GO Microplate Spectrophotometer, Helsinki, Finland). Preliminary screening and cytotoxicity assays were performed in triplicate.
Cell viability assay
Cell viability was assessed using fluorescein diacetate (FDA, Life Technologies (AB & Invitrogen), Carlsbad, CA, USA)-propidium iodide (PI, Life Technologies (AB & Invitrogen)) staining. The cells were incubated with 5 μg ml−1 FDA and 20 μg ml−1 PI in the dark for 5 min. The images were captured using a laser scanning confocal microscope (Nikon A1, Tokyo, Japan).
Cell actin cytoskeleton detection
Actin filaments were stained to observe the cell’s actin cytoskeleton. After culture for 2, 5 and 8 days, the cells were washed with PBS, fixed with 4% paraformaldehyde (Sigma-Aldrich), permeabilized with 0.5% Triton-X (Gibco BRL) and incubated with rhodamine phalloidin (Cytoskeleton, Denver, CO, USA) and Hoechst 33258 (Sigma-Aldrich) in the dark. Cell cytoskeleton organization was observed using a laser scanning confocal microscope.
Alkaline phosphatase activity assay and ALP staining
Alkaline phosphatase (ALP) activity assay and ALP staining were performed using an ALP detection reagent and an ALP staining kit (both purchased from Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China), respectively, according to the manufacturer’s instructions. Culture medium was collected for the ALP activity assay, and the cells were harvested for ALP staining after 2, 5 and 8 days. The absorbance was measured at 520 nm using a microplate reader. All reactions were performed in triplicate. Images of ALP staining were acquired using an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Real-time polymerase chain reaction
Expression of bone morphogenetic protein-2 (BMP2), runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), bone sialoprotein (BSP), osteocalcin (OCN) and alpha-1 type I collagen (COL1A1) was detected using RT-PCR. On days 2, 5 and 8, total RNA was extracted using an RNA extraction kit (Beijing ComWin Biotech, Beijing, China) according to the manufacturer’s instructions. An equal amount of RNA (300 ng) was used as a template for reverse transcription into cDNA using a reverse transcription kit (Fermentas Company, Pittsburgh, PA, USA), and the cDNA was subsequently amplified using a SYBR-Green mix (Roche, Berlin, Germany) and a real-time fluorescence quantitative instrument (Realplex 4, Eppendorf Corporation, Hamburg, Germany). The primers used for PCR are presented in Table 1. Gene expression was analyzed using the 2−ΔΔCT method with β-actin as a control. All samples were assessed in triplicate.
Calcium nodule detection
Calcium nodule detection was performed using Alizarin red staining. After 8 days, the cells were fixed in ice-cold 70% (v/v) ethanol for 1 h, rinsed twice with deionized water and stained with an Alizarin Red S (Sigma-Aldrich) solution (40 mM, pH 4.2) for 10 min at room temperature. Excess dye was gently washed away with running water. Calcification deposits were typically stained red and photographed using an inverted phase contrast microscope.
Protein adsorption study and surface characteristics of BCP
The protein adsorption capacity of BCP was assayed using the BCA protein assay kit (BOSTER, WuHan, China) and calculated according to the calibrated curve using BSA as a standard at 3, 24, 48, 72, 96, 120, 144 and 168 h. All samples were assessed in triplicate. The surface characteristics of BCP were observed using a scanning electron microscope (SEM, SU8020, HITACHI, Tokyo, Japan).
Scanning electron microscope detection
The surface of BCP before and after absorption with NGF was observed using an s.e.m. After 2, 5 and 8 days, the cells on the 3D matrix were also observed using an s.e.m. Briefly, the samples were washed with PBS (pH 7.4) and fixed in 4% paraformaldehyde for 15 min. Subsequently, the fixed samples were gradually dehydrated, incubated in iso-amyl acetate for 5 min and dried. After the substrates were sputter coated with gold, the images were obtained using SEM (VEGA3, TESCAN Company, Brno, Czech Republic).
In vivo experiment
Surgical procedure
Sixty male SD rats weighing 300±20 g were obtained from the Animal Resources Center of Guangxi Medical University (Nanning, Guangxi, China). All experiments were performed in accordance with the relevant guidelines and regulations approved by the Animal Care & Welfare Committee of Guangxi Medical University (Protocol Number: 20141003 A). After randomly dividing the animals into BCP and BCP-NGF groups, with 30 rats per group, the rats were anesthetized with a 2% pentobarbital sodium solution via intraperitoneal injection. Critical-size defects (5 mm in diameter) were created using a dental drill on the right parietal bone. The defects were implanted with BCP pre-immersed in 0 or 2.5 μg ml−1 NGF solution for 7 days. After the surgical experiments, the rats in both groups were intraperitoneally injected with dextrose and normal saline, whereas those in the BCP-NGF group were injected with NGF solution at a dose of 2.5 μg g−1 body weight via the tail vein once a week for 4, 8 and 12 weeks.
Computed tomography imaging analysis, microcomputed tomography analysis and morphological observation
The healing process was observed at 4, 8 and 12 weeks after implantation using cone beam computed tomography (CBCT, Sirona, Galileos, Siemens Aktiengesellschaft, Munich, Germany). After tomographic acquisition, 3D reconstructive images of the samples were generated from 2D slices using machine built-in software. After imaging, the rats were sacrificed, and the entire defective skulls were harvested. Morphological observation was performed to investigate immunoreaction and new bone formation at the defect site. At week 12, the samples were fixed in formalin, scanned and analyzed using microcomputed tomography (micro-CT, Shanghai Showbio Biotech, Shanghai, China).
Histological analysis and immunohistochemical staining
After rinsing with saline, fixing with paraformaldehyde, decalcifying with formic acid and embedding in paraffin, the 5-μm-thick sections were cut through the center of the calvarial defects. For histological analysis, the sections were subjected to hematoxylin and eosin (HE) staining.
For immunohistochemical staining, the sections were heated with citrate buffer for antigen retrieval, blocked with normal blocking serum, and subsequently incubated with mouse anti-osteocalcin (OCN, 1:1000, Santa Cruz, USA) or mouse anti-bone morphogenetic protein-2 (BMP2, 1:1000). After washing three times with PBS, the sections were incubated with goat anti-mouse antibody (1:1000, Santa Cruz, CA, USA), stained with substrate-chromogen solution 3,3′-diaminobenzidine tetrahydrochloride (DAB), and observed using an inverted phase contrast microscope.
Statistical analysis
The values were expressed as the means±s.d. and compared using a one-way analysis of variance (ANOVA). The least significance difference test (LSD-t) was performed for further evaluation of the data. SPSS software (version 16.0, SPSS, Chicago, IL, USA) was used, and P<0.05 was considered a significant difference.
Results
Purification and bioassay of NGF
NGF was extracted from Chinese cobra venom after gel filtration and chromatography through Sephadex G-50, CM Sepharose FF and Superdex 75 PG. The fractions of peak 2 (Figure 1B), peak 5 (Figure 1C) and peak 2 (Figure 1D) were determined based on NGF bioactivity and sequentially collected. The purified NGF was a glycoprotein with molecular weight of 22.3 kDa (Figure 1E). A bioassay with pheochromocytoma PC12 cells revealed the promotion of fiber outgrowth, which is typical of NGF activity (Figure 1F (b)).
Effects of NGF on monolayer-cultured osteoblasts
Cytotoxicity assay
As shown in Figure 2A, preliminary screening revealed that NGF had no significant cytotoxicity at a concentration ranging from 0.625 to 15.625 μg ml−1, but it displayed an inhibitive effect on osteoblasts at concentrations >15.625 μg ml−1 after 3 days of culture. In the range of 1.25 to 5 μg ml−1, NGF notably promoted cell growth. In the present study, concentrations of 2.5, 5 and 10 μg ml−1 were selected for further 2D investigation.
Concentration screening, cytotoxicity assay, FDA-PI staining, rhodamine phalloidin-Hoechst 33258 staining, alkaline phosphatase (ALP) activity assay, ALP staining, osteogenic-specific gene expression and Alizarin red staining of monolayer-cultured osteoblasts. (A) Screening of nerve growth factor (NGF) using various concentrations (0.625, 1.25, 2.5, 5, 7.8125, 10, 15.625, 20, 31.25, 62.5, 125 and 250 μg ml−1) on 2D cultured osteoblasts using the MTT method after 3 days of treatment (n=3). (B) Cytotoxicity assay with NGF at 0, 2.5, 5 and 10 μg ml−1 on days 2, 5 and 8 (n=9). (C (a–d)) FDA-PI staining of osteoblasts treated with NGF at 0, 2.5, 5 and 10 μg ml−1 after 8 days of treatment (Scale bar=200 μm). (C (e–h)) Rhodamine phalloidin-Hoechst 33258 staining of a monolayer culture treated with 0, 2.5, 5 and 10 μg ml−1 NGF after 8 days (Scale bar=100 μm). (D) ALP activity assay of osteoblasts treated with 0, 2.5, 5 and 10 μg ml−1 NGF on days 2, 5 and 8 (n=9). (E (a–d)) ALP staining of osteoblasts treated with 0, 2.5, 5 and 10 μg ml−1 NGF after 8 days (Scale bar=500 μm). (F (a–f)) Relative expression of bone morphogenetic protein-2 (BMP2, F (a)), runt-related transcription factor 2 (RUNX2, F (b)), alkaline phosphatase (ALP, F (c)), bone sialoprotein (BSP, F (d)), osteocalcin (OCN, F (e)) and alpha-1 type I collagen (COL1A1, F (f)) in osteoblasts treated with 0, 2.5, 5 and 10 μg ml−1 NGF on days 2, 5 and 8 (n=3). (G (a–d)) Alizarin red staining of osteoblasts treated with 0, 2.5, 5 and 10 μg ml−1 NGF after 8 days (Scale bar=500 μm). The values are presented as the means±standard deviation. Bars with different letters at the same time point are significantly different from each other at P<0.05, and bars with the same letter exhibit no significant difference.
Further cytotoxicity analysis (Figure 2B) showed that NGF increased cell numbers in a time-dependent manner compared with the control. The cell number increased with time. Among the three concentrations, 5 μg ml−1 NGF exhibited the strongest promoting effect on cell growth.
Cell viability assay and cell actin cytoskeleton detection
Cell viability was determined using FDA-PI staining (Figure 2C (a–d)), in which viable cells stained green and dead cells stained red. Consistent with the results of the cytotoxicity assay, more live cells were observed in the NGF-treated groups compared with the control at each time point, indicating that NGF exerted a positive effect on primary osteoblast survival. Among the experimental groups, the number of viable cells in the 5 μg ml−1 NGF group was superior to that of other groups.
To assess the cytoskeleton reorganization of osteoblasts, actin filaments were detected through rhodamine phalloidin staining. As shown in Figure 2C (e–h), more intensively polymerized actin was observed in the NGF-treated groups compared with the control, and these findings were confirmed using cytotoxicity and cell viability assays. Particularly, 5 μg ml−1 NGF exhibited the greatest induction of the actin cytoskeleton.
ALP activity assay and ALP staining
In all the groups, ALP increased in a time-dependent manner (Figure 2D). Comparatively, osteoblasts in the NGF-treated groups exhibited significantly higher ALP activity than control, which was further confirmed using ALP staining (Figure 2E). The highest ALP level in all the groups was observed with 5 μg ml−1 NGF.
Gene expression
The effect of NGF on osteoblasts was further investigated through an analysis of BMP2, RUNX2, ALP, BSP, OCN and COL1A1 gene expression after 2, 5 and 8 days via RT-PCR assay. As shown in Figure 2F, BMP2, BSP and OCN expression was notably up-regulated by NGF at the three observation points. However, RUNX2, ALP and COL1A1 expression was remarkably up-regulated from day 2 to 5 and subsequently gradually down-regulated thereafter. These osteogenic-specific marker genes exhibited the greatest up-regulation after the administration of 5 μg ml−1 NGF.
Calcium nodules detection
Alizarin red staining was used to detect calcium nodules at day 8. Compared with the control, the formation of calcification deposits was more obvious after NGF treatment, particularly at 5 μg ml−1 (Figure 2G).
Effects of BCP combined with NGF on osteoblasts
Protein adsorption study and surface characteristics of BCP-NGF
In the presence of BCP, the amount of NGF in the solution dramatically decreased after 3 h, and gradually decreased thereafter, becoming stable after 120 h (Figure 3A (a)). In contrast, NGF concentration exhibited minimal variation in the control. This finding indicated that BCP adsorbed the majority of protein in the solution. An s.e.m. was used to investigate the surface of NGF-covered BCP after incubation for 168 h compared with BCP alone (Figure 3A (b)). As shown in Figure 3A (c), protein deposition could be observed on the surface of BCP.
Protein adsorption study and surface characteristics of BCP, NGF concentration screening, cell viability and cell cytotoxicity assays, and cell actin cytoskeleton detection on three-dimensional (3D) cultured osteoblasts. (A (a)) Protein adsorption isotherms of NGF without and with BCP after 3, 24, 48, 72, 96, 120, 144 and 168 h (n=3). (A (b,c)) Scanning electron microscope (s.e.m.) images of BCP combined with NGF at 0 μg ml−1 (b) and 10 μg ml−1 (c) (Scale bar=10 μm, protein deposits are indicated with red arrows). (b) Concentration screening of nerve growth factor (NGF) on three-dimensional (3D) culture using the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) method after 3 days of interference (n=9). (C) Cytotoxicity assay of cells on BCP combined with different concentrations of NGF (0, 1.25, 2.5 and 5 μg ml−1) at days 2, 5 and 8 (n=9). The values are presented as the means±s.d. Bars with different letters at the same time point are significantly different from each other at P<0.05, and bars with the same letter exhibit no significant difference. (D (a–l)) FDA-PI staining of osteoblasts on BCP treated with 0, 1.25, 2.5 and 5 μg ml−1 NGF for 2, 5 and 8 days (Scale bar=100 μm). (E (a–l)) 3D section views of rhodamine phalloidin-Hoechst 33258 staining on BCP combined with 0, 1.25, 2.5 and 5 μg ml−1 NGF treatment for 2, 5, and 8 days (Scale bar=200 μm).
Concentration screening on BCP
As shown in Figure 3B, 0.625 to 2.5 μg ml−1 NGF promoted cell growth, but the doses >2.5 μg ml−1 displayed an inhibitive effect on osteoblasts. In the present study, concentrations of 1.25, 2.5 and 5 μg ml−1 were selected for further 3D investigation.
Cytotoxicity and cell viability assay
As shown in Figure 3C, the cells proliferated in a time-dependent manner. Compared with the control, the cell number increased markedly in the BCP-NGF group, verifying the promoting effect of NGF on 3D culture. Live-dead cell staining was used to determine the effect of NGF on cell viability. As shown in Figure 3D, more viable cells (stained in green) were present in the BCP-NGF group than the BCP alone group (Figure 3D (a). In the BCP-NGF groups, the number of live cells notably increased, and these cells ultimately formed clusters and occupied a majority of the surfaces of the matrices. Among the three doses, 2.5 μg ml−1 NGF promoted cell viability most prominently at all observation points (Figure 3D (c).
Cell cytoskeleton organization
As shown in Figure 3E a section view of 3D image construction revealed that actin polymerization increased with time in all groups. Comparatively, more intensive polymerized actin was distributed throughout the cytoplasm with more aggregation of the cells in the BCP-NGF groups compared with the control. Particularly, 2.5 μg ml−1 NGF exhibited the best performance.
Morphology of cells on BCP
Typical s.e.m. photographs of BCP combined with and without NGF are presented in Figure 4A. A large number of micro-pores (10–100 μm in diameter) distributed on the walls of macro-pores ranging from 100 to 400 μm in diameter could be clearly observed. Cells spread with numerous filamentous extensions in BCP, indicating superior cell attachment to the BCP. More cells were observed in the BCP-NGF group (Figure 4A (k)) than in the control (Figure 4A (a), which ultimately grew in clumps on the surface. This finding indicates the promotion effect of NGF absorbed on BCP, particularly at 2.5 μg ml−1.
Scanning electron microscope (s.e.m.) detection, alkaline phosphatase (ALP) activity assay and gene expression analysis of three-dimensional (3D) cultured osteoblasts. (A (a–l)) Typical s.e.m. photographs of osteoblasts on BCP combined with different concentrations of NGF (0, 1.25, 2.5 and 5 μg ml−1) after 2, 5 and 8 days (Scale bar=100 μm). (B) Relative ALP activity (units/100 ml) in cells on BCP combined with 0, 1.25, 2.5 and 5 μg ml−1 NGF for 2, 5 and 8 days (n=9). (C (a–f)) Relative gene expression of bone morphogenetic protein-2 (BMP2, C (a)), runt-related transcription factor 2 (RUNX2, C (b)), alkaline phosphatase (ALP, C (c)), bone sialoprotein (BSP, C (d)), osteocalcin (OCN, C (e)) and alpha-1 type I collagen (COL1A1, C (f)) on BCP combined with 0, 1.25, 2.5 and 5 μg ml−1 of NGF on days 2, 5 and 8 (n=3). The data are presented as the means±standard deviation. Bars with different letters at the same time point are significantly different from each other at P<0.05, and bars with the same letter exhibit no significant difference.
ALP activity assay
As shown in Figure 4B, increased ALP activity of osteoblasts on BCP combined with NGF was observed from days 2 to 5 compared with the control, particularly at 2.5 μg ml−1. However, ALP activity obviously decreased from days 5 to 8 in all groups. Only ALP activity at 2.5 μg ml−1 NGF was significantly different from the control.
Gene expression
As shown in Figure 4C (a, compared with the control, BMP2, BSP and OCN expression was up-regulated in BCP combined with NGF groups in a time-dependent manner, particularly at 2.5 μg ml−1. However, RUNX2, COL1A1 and ALP expression increased from day 2 to 5 but decreased from day 5 to 8 (Figure 4C (b) in the BCP-NGF groups, and the levels were higher than the control at each time point.
Therapeutic effect of BCP combined with NGF on calvarial-defect model
Imageological examination and gross morphological analysis
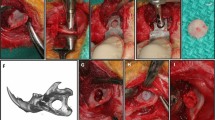
As shown in Figure 5A, the critical calvarial defects were implanted with BCP or BCP pre-immersed in NGF in group BCP or BCP-NGF respectively according to the model. One rat in the BCP group died at one week post-operation, which may reflect brain damage or infection. In addition, another rat in group BCP-NGF died during anesthesia, which may reflect excessive anesthetic. The remaining rats recovered well after operation and remained alive until the end of the study. After 4, 8 and 12 weeks of therapy, the samples were harvested for observation and analyses. As shown in Figure 5, no inflammation was observed in the engineered bone at weeks 4, 8 and 12. Imageological examination (Figures 5B and D (b)) and gross morphology observation (Figure 5C) showed the nearly complete repair of bone defects using combinational therapy. The new bone was remodeled and fused with the surrounding tissue after 12 weeks (Figure 5E), as evidenced by significant increases in parameters, including bone volume (BV, Figure 5E (b)) and the percentage of bone volume/total volume (BV/TV, Figure 5E (c)). Trabecular analyses were also significantly improved with NGF treatment, including an obvious increase in trabecular thickness (Tb.Th, Figure 5E (d)) and trabecular number (Tb.N, Figure 5E (e)), and a notable decrease in trabecular separation (Tb.Sp, Figure 5E (f)). However, the interface between the neo-bone and the original tissue remained obvious in the BCP-repaired defects, indicating less favorable bridging. In summary, all the data suggested that combinational therapy of BCP and NGF was superior to treatment with BCP alone.
Imageological examination and gross morphological analysis of repaired calvarial tissue in the calvarial-defect model. (A) The critical calvarial-defect model with BCP implanted during the operation. (B) Cone beam CT scan of calvarial defects repaired by BCP combined without (B (a,c,e)) or with NGF therapy (B (a,d,f)) after 4, 8 and 12 weeks. (c) Gross morphological observation of calvarial defects repaired by BCP combined without (C (a,c,e)) or with NGF therapy (C (b,d,f)) after 4, 8 and 12 weeks. (D) Representative three-dimensional micro-CT reconstructions of calvarial defects repaired by BCP combined without (D (a)) or with NGF therapy (D (b)) after 12 weeks. (E) Micro-CT quantification data, including bone mineral density (BMD, E (a)), bone volume (BV, E (b)), bone volume/total volume (BV/TV, E (c)), trabecular thickness (Tb.Th, E (d)), trabecular number (Tb.N, E (e)), and trabecular separation (Tb.Sp, E (f)). n=3 samples per group. * indicates P<0.05, ** indicates P<0.01. micro-CT, microcomputed tomography.
Histological and immunohistochemical analysis
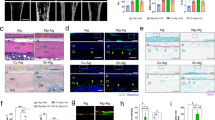
HE staining (Figure 6A) showed that new bone formed in the defect near the original bone, which became more uniform and compact as time progressed. Compared with bone repair by BCP alone, enhanced bone ingrowth with relatively more positive OCN (Figure 6B) and BMP2 (Figure 6C) staining was observed with combinational therapy. The boundary between the new bone and the original tissue was more compact in BCP and NGF therapy than with BCP alone.
Hematoxylin–eosin (HE) staining and immunohistochemical staining on sections of repaired calvarial tissue. (A (a–f)) HE staining of repaired calvarial tissue from a calvarial-defect model implanted with BCP combined without or with NGF at weeks 4, 8 and 12 (Scale bar=100 μm in the large picture and Scale bar=1000 μm in the small picture). (B (a–f)) Osteocalcin (OCN) immunostaining of repaired calvarial tissue from the calvarial-defect model implanted with BCP combined without or with NGF at weeks 4, 8 and 12 (Scale bar=100 μm in the large picture and Scale bar=1000 μm in the small picture). (C (a–f)) Bone morphogenetic protein-2 (BMP2) immunostaining of repaired calvarial tissue from a calvarial-defect model implanted with BCP combined with or without NGF at week 4, 8 and 12 (Scale bar=100 μm in the large picture and Scale bar=1000 μm in the small picture).
Discussion
BCP concentrates proteins on the surface, and this combination exerts synergistic effects on cell behavior. In the present study, we demonstrated that BCP combined with NGF significantly promoted the growth of osteoblasts and bone regeneration, and this system may be developed as a potential agent for skeletal reconstruction.
Regarding evidence for neuro-osteological interactions, NGF is pivotal not only in the maintenance and regeneration of nerves but also in the stimulation of the differentiation and inhibition of apoptosis of osteoblastic cells.14 The results of 2D culture in the present study showed that NGF greatly promoted osteoblast growth and differentiation, with more viable cells and higher ALP activity than the control (Figures 2C and E). After loading in BCP, the growth of osteoblasts and new bone formation were extensively increased, based on morphological, histological and PCR findings with increased markers for osteogenesis compared with either NGF or BCP alone. These findings indicate that BCP facilitates angiogenesis and osteoconduction, and NGF enables neurogenesis, both of which contribute to new bone formation.
An important feature of Ca-P ceramics is protein adsorption,34, 35 which favors the concentration of proteins in a natural manner. In the present study, NGF was immobilized on the surface of BCP, which was minimally released, even after 7 days of incubation. Interestingly, optimal NGF doses substantially differed between the monolayer and 3D cultures at 2.5–5 μg ml−1 and 1.25–2.5 μg ml−1, respectively. In particular, 5 μg ml−1 NGF best supports cell growth in 2D culture, whereas 2.5 μg ml−1 NGF was better than other doses in 3D culture, indicating that BCP selectively absorbed NGF in the culture medium, leading to the increased local concentration of NGF at the BCP surface.
Further exploration revealed that the combination of BCP and NGF promoted osteoblast differentiation through the regulation of the BMP2/Runx2 signaling pathway. This signaling has been identified as one of the major pathways for osteogenesis41 and controls the downstream gene Runx2 through the BMP signaling pathway.42, 43, 44 BMP2 exhibits a time-dependent increase after treatment with BCP and NGF (Figures 2F (a) and 4C (a)), indicating that osteoblast differentiation may be mediated by BMP2 signaling. As a downstream transcription factor, Runx2 increases at early stages and subsequently declines. The results were consistent with those of a previous study45 demonstrating that the overexpression of Runx2 in the late stage disfavors osteoblast maturation.46, 47 Animal models of calvarial reconstruction also confirmed this finding, demonstrating increased BMP2 expression as a result of combinational therapy.
Another advantage of this combinational therapy is the low cost and ease of accessibility. Unlike other growth factors, NGF has abundant sources48 and NGF isolation and purification methods are well established and simple for industrialization. Using the optimized three-step procedure described herein, purified and bioactive NGF was easily extracted from Chinese cobra venom. Similarly, BCP is also easily produced.
In conclusion, porous BCP combined with NGF exerts potent effects on osteoblast differentiation and calvarial regeneration, and the effects of combination treatment were superior to either treatment alone. The results confirmed the neuro-osteological interactions in the promotion of osteogenesis and acceleration of bone formation.
Change history
05 July 2019
A Correction to this paper has been published: https://doi.org/10.1038/s12276-019-0270-y
27 January 2020
A Correction to this paper has been published: https://doi.org/10.1038/s12276-020-0378-0
20 January 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s12276-023-00931-3
References
Chung SM, Jung IK, Yoon BH, Choi BR, Kim DM, Jang JS . Evaluation of different combinations of biphasic calcium phosphate and growth factors for bone formation in calvarial defects in a rabbit model. Int J Periodontics Restorative Dent 2016; 36: s49–s59.
Mangano C, Sinjari B, Shibli JA, Mangano F, Hamisch S, Piattelli A et al. A human clinical, histological, histomorphometrical and radiographical study on biphasic HA-Beta-TCP 30/70 in maxillary sinus augmentation. Clin Implant Dent Relat Res 2015; 17: 610–618.
Hu J, Yang Z, Zhou Y, Liu Y, Li K, Lu H . Porous biphasic calcium phosphate ceramics coated with nano-hydroxyapatite and seeded with mesenchymal stem cells for reconstruction of radius segmental defects in rabbits. J Mater Sci Mater Med 2015; 26: 257.
de Gabory L, Delmond S, Deminiere C, Stoll D, Bordenave L, Fricain JC . Assessment of biphasic calcium phosphate to repair nasal septum defects in sheep. Plast Reconstr Surg 2011; 127: 107–116.
Bodde EW, Wolke JG, Kowalski RS, Jansen JA . Bone regeneration of porous beta-tricalcium phosphate (Conduit TCP) and of biphasic calcium phosphate ceramic (Biosel) in trabecular defects in sheep. J Biomed Mater Res A 2007; 82: 711–722.
Farina NM, Guzon FM, Pena ML, Cantalapiedra AG . In vivo behaviour of two different biphasic ceramic implanted in mandibular bone of dogs. J Mater Sci Mater Med 2008; 19: 1565–1573.
Roldan JC, Detsch R, Schaefer S, Chang E, Kelantan M, Waiss W et al. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J Craniomaxillofac Surg 2010; 38: 423–430.
Kjaer I . Neuro-osteology. Crit Rev Oral Biol Med 1998; 9: 224–244.
Persson E, Lerner UH . The neuropeptide VIP potentiates IL-6 production induced by proinflammatory osteotropic cytokines in calvarial osteoblasts and the osteoblastic cell line MC3T3-E1. Biochem Biophys Res Commun 2005; 335: 705–711.
Lerner UH, Persson E . Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Musculoskelet Neuronal Interact 2008; 8: 154–165.
Lomholt JF, Keeling JW, Hansen BF, Ono T, Stoltze K, Kjaer I . The prenatal development of the human cerebellar field in Down syndrome. Orthod Craniofac Res 2003; 6: 220–226.
Letic-Gavrilovic A, Piattelli A, Abe K . Nerve growth factor beta(NGF beta) delivery via a collagen/hydroxyapatite (Col/HAp) composite and its effects on new bone ingrowth. J Mater Sci Mater Med 2003; 14: 95–102.
Wang L, Cao J, Lei DL, Cheng XB, Zhou HZ, Hou R et al. Application of nerve growth factor by gel increases formation of bone in mandibular distraction osteogenesis in rabbits. Br J Oral Maxillofac Surg 2010; 48: 515–519.
Cao J, Wang L, Lei DL, Liu YP, Du ZJ, Cui FZ . Local injection of nerve growth factor via a hydrogel enhances bone formation during mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol oral Radiol 2012; 113: 48–53.
Arumäe U, Neuman T, Sinijärv R, Saarma M . Sensitive time-resolved fluoroimmunoassay of nerve growth factor and the disappearance of nerve growth factor from rat pheochromocytoma PC12 cell culture medium. J Immunol Methods 1989; 122: 59–65.
Lipps BV . Biological and immunological properties of nerve growth factor from snake venoms. J Nat Toxins 1998; 7: 121–130.
Lipps BV . Detection of nerve growth factor (NGF) in venoms from diverse source: isolation and characterization of NGF from the venom of honey bee (Apis melifera). J Nat Toxins 2000; 9: 13–19.
Lipps BV . Isolation of nerve growth factor (NGF) from human body fluids; saliva, serum and urine: comparison between cobra venom and cobra serum NGF. J Nat Toxins 2000; 9: 349–356.
Lipps BV, Khan AA., Lipps BV . Antigenic cross reactivity among the venoms and toxins from unrelated diverse sources. Toxicon 2000; 38: 973–980.
Lipps BV . Isolation of subunits, alpha, beta and gamma of the complex taipoxin from the venom of Australian taipan snake (Oxyuranus s. scutellatus: characterization of beta taipoxin as a potent mitogen. Toxicon 2000; 38: 1845–1854.
Mariam K, Tu AT . Extremely low nerve growth facior (NGF) activity of sea snake (Hydrophiidae) venoms. J Nat Toxins 2002; 11: 393–398.
Asanome A, Kawabe J, Matsuki M, Kabara M, Hira Y, Bochimoto H et al. Nerve growth factor stimulates regeneration of perivascular nerve and induces the maturation of microvessels around the injured artery. Biochem Biophys Res Commun 2014; 443: 150–155.
Chen WH, Mao CQ, Zhuo LL, Ong JL . Beta-nerve growth factor promotes neurogenesis and angiogenesis during the repair of bone defects. Neural Regen Res 2015; 10: 1159–1165.
Grills BL, Schuijers JA, Ward AR . Topical application of nerve growth factor improves fracture healing in rats. J Orthop Res 1997; 15: 235–242.
Wang L, Zhou S, Liu B, Lei D, Zhao Y, Lu C et al. Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J Orthop Res 2006; 24: 2238–2245.
Dodla MC, Bellamkonda RV . Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008; 29: 33–46.
Lee JY, Lee JW, Schmidt CE . Neuroactive conducting scaffolds: nerve growth factor conjugation on active ester-functionalized polypyrrole. J R Soc Interface 2009; 6: 801–810.
Lee JY, Bashur CA, Milroy CA, Forciniti L, Goldstein AS, Schmidt CE . Nerve growth factor-immobilized electrically conducting fibrous scaffolds for potential use in neural engineering applications. IEEE Trans Nanobiosci 2012; 11: 15–21.
de Boer R, Knight AM, Borntraeger A, Hebert-Blouin MN, Spinner RJ, Malessy MJ et al. Rat sciatic nerve repair with a poly-lactic-co-glycolic acid scaffold and nerve growth factor releasing microspheres. Microsurgery 2011; 31: 293–302.
Jin J, Limburg S, Joshi SK, Landman R, Park M, Zhang Q et al. Peripheral nerve repair in rats using composite hydrogel-filled aligned nanofiber conduits with incorporated nerve growth factor. Tissue Eng Part A 2013; 19: 2138–2146.
Zhang H, Wang K, Xing Y, Yu Q . Lysine-doped polypyrrole/spider silk protein/poly(l-lactic) acid containing nerve growth factor composite fibers for neural application. Mater Sci Eng C Mater Biol Appl 2015; 56: 564–573.
Chao X, Xu L, Li J, Han Y, Li X, Mao Y et al. Facilitation of facial nerve regeneration using chitosan-beta-glycerophosphate-nerve growth factor hydrogel. Acta Otolaryngol 2016; 136: 585–591.
Esfahani H, Prabhakaran MP, Salahi E, Tayebifard A, Rahimipour MR, Keyanpour-Rad M et al. Electrospun nylon 6/zinc doped hydroxyapatite membrane for protein separation: mechanism of fouling and blocking model. Mater Sci Eng C Mater Biol Appl 2016; 59: 420–428.
Zhu XD, Zhang HJ, Fan HS, Li W, Zhang XD . Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater 2010; 6: 1536–1541.
Wang J, Zhang H, Zhu X, Fan H, Fan Y, Zhang X . Dynamic competitive adsorption of bone-related proteins on calcium phosphate ceramic particles with different phase composition and microstructure. J Biomed Mater Res B Appl Biomater 2013; 101: 1069–1077.
Wang J, Chen Y, Zhu X, Yuan T, Tan Y, Fan Y et al. Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. J Biomed Mater Res A 2014; 102: 4234–4243.
Alam I, Asahina I, Ohmamiuda K, Enomoto S . Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res 2001; 54: 129–138.
Kim BS, Lee J . Enhanced bone healing by improved fibrin-clot formation via fibrinogen adsorption on biphasic calcium phosphate granules. Clin Oral Implants Res 2015; 26: 1203–1210.
Li XB, Chen MJ, Lei DQ, Yang B, Liao GS, Shu YY et al. Bioactivities of nerve growth factor from Chinese cobra venom. J Nat Toxins 1999; 8: 359–362.
Li XB, Liao GS, Shu YY, Tang SX . Brain delivery of biotinylated NGF bounded to an avidin–transferrin conjugate. J Nat Toxins 2000; 9: 73–83.
Benisch P, Schilling T, Klein-Hitpass L, Frey SP, Seefried L, Raaijmakers N et al. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS ONE 2012; 7: e45142.
Hanai J, Chen LF, Kanno T, Ohtani-Fujita N, Kim WY, Guo WH et al. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Calpha promoter. J Biol Chem 1999; 274: 31577–31582.
Javed A, Barnes GL, Jasanya BO, Stein JL, Gerstenfeld L, Lian JB et al. runt homology domain transcription factors (Runx, Cbfa, and AML) mediate repression of the bone sialoprotein promoter: evidence for promoter context-dependent activity of Cbfa proteins. Mol Cell Biol 2001; 21: 2891–2905.
Nishimura R, Hata K, Harris SE, Ikeda F, Yoneda T . Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone 2002; 31: 303–312.
Jin P, Wu H, Xu G, Zheng L, Zhao J . Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study. Cell Tissue Res 2014; 356: 381–390.
Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol 2001; 155: 157–166.
Cobb J, Dierich A, Huss-Garcia Y, Duboule D . A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc Natl Acad Sci USA 2006; 103: 4511–4515.
Kilmon J . Snake venom: 'gentler' purification provides attractive nerve growth factor source. Am Biotechnol Lab 1992; 10: 18–20.
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (grant no. 2016YFB0700804), the Guangxi Scientific Research and Technological Development Foundation (grant no. Guikegong 1598013-15) and the Guangxi Science Fund for Distinguished Young Scholars (grant no. 2014GXNSFGA118006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jin, P., Yin, F., Huang, L. et al. RETRACTED ARTICLE: Guangxi cobra venom-derived NGF promotes the osteogenic and therapeutic effects of porous BCP ceramic. Exp Mol Med 49, e312 (2017). https://doi.org/10.1038/emm.2016.173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2016.173
This article is cited by
-
Effects of exogenous nerve growth factor on the expression of BMP-9 and VEGF in the healing of rabbit mandible fracture with local nerve injury
Journal of Orthopaedic Surgery and Research (2021)