Abstract
Study design:
Retrospective study.
Objectives:
We aimed to describe the epidemiology of multidrug-resistant organisms (MDROs) during bloodstream infection (BSI) and identify associated risks of MDROs among patients with spinal cord injury (SCI).
Setting:
A teaching hospital, expert center in disability, in France.
Methods:
We studied a retrospective cohort of all BSIs occurring in SCI patients hospitalized over 16 years. We described the prevalence of MDRO BSI among this population and its evolution over time and compared the BSI population due to MDROs and due to non-MDROs.
Results:
A total of 318 BSIs occurring among 256 patients were included in the analysis. The most frequent primary sites of infection were urinary tract infection (34.0%), pressure sore (25.2%) and catheter line-associated bloodstream infection (11.3%). MDROs were responsible for 41.8% of BSIs, and this prevalence was stable over 16 years. No significant associated factor for MDRO BSI could be identified concerning sociodemographic and clinical characteristics, primary site of infection and bacterial species in univariate and multivariate analyses. BSI involving MDROs was not associated with initial severity of sepsis compared with infection without MDROs (43.8 vs 43.6%, respectively) and was not associated either with 30th-day mortality (6.2 vs 9%, respectively).
Conclusion:
During BSI occurrence in an SCI population, MDROs are frequent but remain stable over years. No associated risk can be identified that would help optimize antibiotic treatment. Neither the severity of the episode nor the mortality is significantly different when an MDRO is involved.
Similar content being viewed by others
Introduction
Approximately 276 000 people are living with spinal cord injury (SCI) in the United States, with an annual incidence of 12 500 new cases each year.1 In Europe, the incidence of SCI is between 10.4 and 29.4 per million habitants. About half of the SCIs have a traumatic etiology.2 People with SCI have an increased risk of systemic infection, pneumonia and septicemia, which cause most infection-related deaths (~35%).3, 4, 5 Blood stream infections (BSIs) are more common in this population than in the general population.6, 7, 8 In literature, the most common primary site for BSI in patients with SCI is the urinary tract.3, 9 Frequently reported causative organisms of BSI in this population are Staphylococcus aureus and Gram-negative bacteria.3, 7, 8, 9, 10, 11 Currently, the increase in drug-resistant bacteria in recent years underlines the importance of gathering accurate microbiological information.3 Colonizing multidrug-resistant organisms (MDROs) are frequent in the SCI population12, 13, 14 and can be due to the massive antibiotic exposure and frequent and prolonged hospitalization.7, 15, 16
In the general population, hospital-acquired infections and MDRO infections are associated with increased mortality and increased cost.7 In the SCI population, such infections have been shown to increase the number of hospitalizations and the length of stay.17 Data on MDRO infection among the SCI population are scarce. The aim of our study was to describe the prevalence of MDRO bacteria during BSI episodes in patients with SCI and to compare the characteristics of the MDRO population with the non-MDRO population. We developed a large comparative study and looked for associated risks.
Materials and methods
Settings and design
We performed a retrospective comparative study with medical chart review over a 16-year period (from 1 July 1998 to 31 October 2013) among all patients hospitalized at the Raymond Poincare Teaching Hospital, Garches, Paris, France, that had an international classification disease 10th version (ICD-10) such as paraplegic or quadriplegic and positive blood cultures for bacteria.
The microbiological data were obtained from the local computerized medical entry, and medical charts were reviewed to collect demographic characteristics, associated risks and outcomes.
Population
SCI patients were identified by code ICD-10, and medical charts were reviewed to exclude code mistakes.
Bacterial BSI was defined as follows:
-
At least one positive blood culture. For common skin contaminants (for example, coagulase-negative Staphylococci, Corynebacteria and so on) at least two different sets of blood cultures were required.
-
A prescription of a systemic antibiotic treatment to treat the BSI.
Polymicrobial BSI was defined as having more than one organism found in the same BSI episode.
Definitions
Microbiological definitions
During the period of the study, the identification methods have changed. Until May 2011, Gram-negative bacilli, except for Pseudomonas aeruginosa and Stenotrophomonas maltophilia, were phenotypically identified by the API 20E biochemical identification system (BioMérieux, Lyon, France). P. aeruginosa and S. maltophilia were identified by the API 20 NE biochemical identification system (BioMérieux). The identification of Staphylococci was based on the coagulase test (Bio-Rad, Paris, France), and the identification of Enterococci was based on the use of tellurite and bile esculin agar media (BD Diagnostics, Sparks, NV, USA). After May 2011, all isolates were identified by mass spectrometry (Bruker, Bremen, Germany).
Antimicrobial susceptibility testing was carried out using the agar disk diffusion method (Bio-Rad) or an automated broth microdilution method (Phoenix, BD Diagnostics, Oxford, UK). The breakpoints used were those defined by the French Committee for Antimicrobial Susceptibility Testing (CA-SFM).
The MDRO status was asserted after reviewing the antibiotic sensitivity and comparing it with the definitions mentioned in Table 1.
Confirmation of extended-spectrum β-lactamase (ESBL) activity was provided by testing cefotaxime and ceftazidime in the presence and absence of clavulanic acid, isolates for which the minimum inhibitory concentration of cefotaxime or ceftazidime decreased by 3 twofold dilutions when tested in the presence of clavulanic acid or for which zone diameters increased by 5 mm in the presence of clavulanic acid were considered positive for ESBL, as described by the National Committee for Clinical Laboratory Standards.18, 19
Primary site of infection
Primary site of infection was defined as clinically suspected (by the physician in charge or reported on the medical chart) or bacteriologically documented with the same bacterial identification as that in the blood culture. Primary sites were categorized as urinary tract infection (UTI), pressure sore, catheter line-associated BSI, osteoarticular infection, pulmonary tract infection, other (including skin and soft tissue infection and intra-abdominal infection) and unknown when no primary site had been identified.
Severity
Severity was defined as the requirement of at least one of the following criteria: volume expansion required, assisted (mechanical) ventilation, vasopressor requirement and ICU (intensive care unit) admission during the episode.
Mortality
Mortality was defined as dead status before 30 days.20
Occurrence of MDROs and non-MDROs over years
To evaluate the proportion of MDROs according to time, the duration of the study was divided into 4-year periods (1998–2001; 2002–2005; 2006–2009; 2010–2013). For each period, percentages of MDROs were collected and compared with each other.
Statistical analysis
All continuous variables are presented as mean and s.d., and the categorical variables are presented as frequencies. Correlations between risk factors and characteristics of BSI in patients with SCI and outcome (mortality and severity) were determined by Student’s t-test for continuous variables and the Pearson's χ2 test for categorical variables.
Logistic regression analysis was performed to assess the relationship between all associated risks that had a P-value⩽0.2 in the bivariate analysis as independent variables and MDROs as the dependent variable. The Hosmer–Lemeshow statistic was calculated to assess the model’s goodness of fit.
The relative risks of MDROs were estimated by calculating the adjusted odds ratios and corresponding 95% confidence intervals. Odds ratios were adjusted for all genders and ages to control for increased risk of MDROs. All reported probability values (P-values) were based on two-sided tests, and a P-value<0.05 was considered statistically significant. All analyses were performed using the Statistical Package for Social Science (SPSS) version 17.0 (SPSS, Chicago, IL, USA).
Results
Population
From 1 July 1998 to 31 October 2013, 396 episodes of positive blood cultures were detected. Seventy-eight episodes were dismissed because they were considered to be contaminated; hence, 318 episodes of BSI were included among 256 patients. Table 2 shows the sociodemographic and clinical characteristics of non-MDRO and MDRO populations.
The median age of the cohort was 50.84 (±17.04) years, and the sex ratio was 0.73; 156 patients (61%) of the global population were paraplegic and 100 (39%) tetraplegic. The mean duration of SCI was 11.6 years.
The most common cause of SCI was mainly post-traumatic (n=177; 55%) including motor vehicular accidents, acts of violence, falls and sporting accidents, followed by multiple sclerosis (n=31; 10%) and infection (n=15; 5%).
Other origins, which accounted for 86 cases (27%), included vascular insult (for example, medullary ischemia), autoimmune disease (other than multiple sclerosis), spinal disc herniation and lumbar-canal stenosis.
Primary site of infection
The primary site of infection was identified in 91% of cases, including 34% with UTI (n=108), followed by pressure sore (n=80; 25.2%), catheter line-associated BSI (n=36; 11.3%), pulmonary tract infection (n=27; 8.5%) and osteoarticular infection (n=19; 6%).
Bacteria and resistance
In total, 351 micro-organisms were isolated in 318 BSI episodes; 31 (9.7%) were polymicrobial (Table 3).
The most causative pathogens were Enterobacteriaceae, which were identified in 42.5% of cases (n=135), followed by S. aureus (n=84; 26.4%), P. aeruginosa (n=11; 3.5%), Streptococcus spp. (n=32; 10.1%) and Enterococcus spp. (n=16; 5%).
Distributions of bacteria depending on primary sites of infection and outcomes are shown in Tables 3 and 4, respectively.
MDROs were involved in 40.5% of BSI and were distributed as follows: 50.4% Enterobacteriacae (E. coli (n=28), Klebsiella spp. (n=13), Proteus spp. (n=8), Enterobacter spp. (n=5), Morganella spp. (n=4), Citrobacter spp. (n=3), Providencia spp. (n=2) and Serratia spp. (n=1)), 34.9% S. aureus (MRSA) and 6.1% P. aeruginosa.
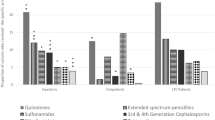
The proportion of MDROs over time was high but stable considering the four periods even when we focus on subgroups such as MRSA, MDRO enterobacteria or ESBL enterobacteria producers (Figure 1). Actually, despite some variations, the prevalence of MDROs, ESBL producers and MRSA was not significantly different according to time (P-values are, respectively, 0.155, 0.553 and 0.159).
No cases of carbapenemase-producing bacteria or vancomycin-resistant Enterocci were reported during this period.
Severity and mortality
At initial presentation, 43.4% of episodes were considered to be severe, as defined by ICU admission (34.9%), volume expansion (24.8%), mechanical ventilation (19.8%) and vasopressor requirements (15.7%). The mortality rate was 9.1%.
When looking at the association between initial signs of severity and overall mortality, we found that an episode that was initially severe was associated with a high mortality rate (P-value of 0.0001 and odds ratio of 5.73 (2.26–14.51)).
Comparing populations with BSI due to MDROs with non-MDROs
No significant difference was observed between MDRO and non-MDRO groups regarding sociodemographic and clinical characteristics (such as age, gender, paraplegia/tetraplegia criteria, primary site of infection, severity and mortality; Table 2).
No association was found in univariate and multivariate analyses between MDROs and age, gender, paraplegia/tetraplegia criteria, duration of SCI, presence of UTI and initial severity (Table 5).
Discussion
Few studies have reported the incidence of MDROs during BSI among the SCI population despite its potentially life-threatening nature. Most recent published data are from the 2000s.6, 7, 9 It seems most important to us to evaluate this topic in the era of the emergence of bacterial resistance worldwide.
Our study population
When considering the occurrence and type of BSI in an SCI population, a difference should be made between recent SCI and chronic SCI. In a retrospective study by Waites et al.9 of hospitalized people with SCI, the microbiology of BSI during initial vs subsequent hospitalizations was compared. The respiratory tract is the origin of the majority of BSIs during recent SCI, and the urinary tract is the primary origin during rehospitalizations. UTI is still a major diagnosis challenge in SCI patients, despite newly developed techniques such as IL8 dosage, which is an effective biological marker of infection, especially UTI. 21
In our study, patients have chronic SCI and the characteristics of the studied population taking into consideration age, sex ratio, proportion of paraplegic/tetraplegic and cause of SCI (traumatism, multiple sclerosis, neoplasia, infection, idiopathic, vascular and so on; Table 2) are the same as other previous reports on a chronic SCI population.3, 9, 11
Bacteria
With regard to involved bacteria, most of the authors state that Gram-negative bacteremia is the most common in this situation, and the primary site of infection is in most of the cases a UTI due to bladder dysfunction.3, 8, 9, 10, 11, 22, 23 These previous results corroborate our study, where the most frequent primary sites are UTI and pressure sore as commonly described in the literature, and Gram-negative bacteria are the most frequently involved micro-organisms followed by S. aureus (Tables 2 and 3).3, 11, 24 Polymicrobial infection is not rare and is mostly due to pressure sore.
Prevalence/incidence of MDROs in BSI among SCI population
There is an increasing incidence of infections due to MDROs in the general population, especially with Enterobacteriacae and particularly ESBL producers and now carbapenemase producers.25
In the general population, infections due to MDROs are associated with an increased length of stay in hospital, an increased cost and an increased mortality.7
Few data are available about the prevalence of infections due to MDROs among the SCI population.
Waites et al.26 report an antimicrobial resistance of about 33% from urine specimens in outpatients with SCI. Mylotte et al.14 also found a large proportion (42%) of MDROs in nosocomial infections in an SCI population.
In our large study, considering infection and not colonization, the prevalence of MDROs is high but remains stable, whatever bacteria are involved (P. aeruginosa, Klebsiella spp., E. coli, Proteus spp., S. aureus and so on; Figure 1).
Hence, the prevalence of MDROs in the SCI population is stable, whereas it is increasing in the general population. This discordance may be due to the already high proportion of MDROs in this specific population because of its already high exposure. Moreover, it is difficult to measure an increasing prevalence as it is already so high.
Spinal cord-injured patients are often exposed to antimicrobial therapy due to the higher incidence of infections in general and UTI in particular. This high antibiotic exposure leads to a higher MDRO incidence. Thus, original strategies to prevent infection without antimicrobials are of most interest. The use of non-virulent bacterial strains, for example, is one mean to protect the patients against UTI.27
Associated risks of MDROs in BSI
The main risks factors for the acquisition of bacterial drug resistance are well known: age, recent hospitalization, previous antibiotic treatment, which is the biggest risk factor, and the presence of a catheter line or urinary device. Some authors have also identified chronic disease and immunosuppression, functional status and APACHE score as risk factors.28, 29, 30, 31, 32, 33, 34
Few studies focus on the SCI population, which is most exposed to antibiotics.28
We looked for a connection between the characteristics of infection and the presence of MDROs during BSI. Identifying associated factors would help physicians predict MDRO infection and optimize the first-line antibiotic treatment. Unfortunately, no associated risk regarding age, gender, duration of SCI, primary site of infection or bacteria species has been identified in this population.
Severity
We report a high proportion of episodes with severe presentation, mostly due to ICU admission and volemic expansion. This high rate of severe presentation has not yet been reported. Only Bhatt et al.10 in their study report hypotension in 5 out of 29 cases (17.1%).
There is no significant difference between MDRO and non-MDRO populations in terms of the proportion of severe presentation.
Mortality
In the general population, the usual rate of mortality varies from 30% to 50%.35, 36 In the SCI population, according to several reports it varies from 1.7% to 29%.6, 9, 10, 11
In our data, the mortality rate is low (9.1%). Several hypotheses are usually advanced to explain this situation: the generally younger age of the SCI population, the lack of underlying illness and the possibility of a particular immunity due to recurrent infections such as UTI and the development of antibodies against bacteremia.10, 11, 15
Furthermore, in our experience, there is no difference between MDRO and non-MDRO populations regarding mortality. This could be explained by the metabolic cost-effectiveness of bacterial resistance that makes bacteria less virulent.37, 38, 39
Bias and weakness
The bias and weakness of our study are due to its monocentric and retrospective design. However, it is one of the largest cohorts of patients in relation to this topic to the best of our knowledge. Therefore, these retrospective results need to be confirmed by future prospective studies. The definition of MDROs could be discussed, but there is no standard definition of antimicrobial resistance, especially for P. aeruginosa and other Gram-negative bacilli. Thus, we used a classification proposed by different authors.18, 19, 40, 41, 42, 43
With regard to BSI in SCI patients, UTI and decubitus ulcer/pressure sore are the main primary sites of infection. The main bacteria involved are Gram-negative bacteria and Staphylococci.
MDROs are frequent in BSI occurring in the SCI population but remain stable over 16 years. There is no significant difference regarding main clinical characteristics, primary sites of infection and bacterial species comparing the MDRO with the non-MDRO population in univariate and multivariate analysis.
The MDRO character of the bacteria involved has no impact on either the severity or the mortality of the episode.
Data Archiving
There were no data to deposit.
References
National Spinal Cord Injury Statistical Center Spinal Cord Injury Facts and Figures at a Glance 2015. National Institute on Disability and Rehabilitation: Washington, USA, Available at https://www.nscisc.uab.edu/PublicDocuments/reports/pdf/2015%20NSCISC%20Annual%20Statistical%20Report%20Complete%20Public%20Version.pdf (accessed on 21 August 2015).
Haute Autorité de Santé. Guide Affection Longue Durée - Paraplégie (lésions médullaires) 2007.
Wall BM, Mangold T, Huch KM, Corbett C, Cooke CR . Bacteremia in the chronic spinal cord injury population: risk factors for mortality. J Spinal Cord Med 2003; 26: 248–253.
DeVivo MJ, Stover SL Long term survival and causes of death. In: Stover SL, DeLisa JA, Whiteneck GG (eds). Spinal Cord Injury: Clinical Outcomes from the Model Systems. Aspen Publishers: Gaithersburg, MD, USA. 1995, pp 289–313.
DeVivo MJ, Krause JS, Lammertse DP . Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999; 80: 1411–1419.
Darouiche RO Infection and spinal cord injury. In: Lin VW, Cardenas DD, Cutter NC, Frost FS, Hammond MC, Lindblom LB et al (eds). Spinal Cord Medicine: Principles and Practice. Demos Medical Publishing, Inc.: New York, NY, USA. 2003, pp 201–220.
Evans CT, LaVela SL, Weaver FM, Priebe M, Sandford P, Niemiec P et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol 2008; 29: 234–242.
Evans CT, Hershow RC, Chin A, Foulis PR, Burns SP, Weaver FM . Bloodstream infections and setting of onset in persons with spinal cord injury and disorder. Spinal Cord 2009; 47: 610–615.
Waites KB, Canupp KC, Chen Y, DeVivo MJ, Moser SA . Bacteremia after spinal cord injury in initial versus subsequent hospitalizations. J Spinal Cord Med 2001; 24: 96–100.
Bhatt K, Cid E, Maiman D . Bacteremia in the spinal cord injury population. J Am Paraplegia Soc 1987; 10: 11–14.
Montgomerie JZ, Chan E, Gilmore DS, Canawati HN, Sapico FL . Low mortality among patients with spinal cord injury and bacteremia. Rev Infect Dis 1991; 13: 867–871.
Maeder K, Ginunas VJ, Montgomerie JZ, Canawati HN . Methicillin-resistant Staphylococcus aureus (MRSA) colonization in patients with spinal cord injury. Paraplegia 1993; 31: 639–644.
Thom JD, Wolfe V, Perkash I, Lin VW . Methicillin-resistant Staphylococcus aureus in patients with spinal cord injury. J Spinal Cord Med 1999; 22: 125–131.
Mylotte JM, Kahler L, Graham R, Young L, Goodnough S . Prospective surveillance for antibiotic-resistant organisms in patients with spinal cord injury admitted to an acute rehabilitation unit. Am J Infect Control 2000; 28: 291–297.
Montgomerie JZ . Infections in patients with spinal cord injuries. Clin Infect Dis 1997; 25: 1282–1285.
Vincent JL . Nosocomial infections in adult intensive-care units. Lancet 2003; 361: 2068–2077.
LaVela SL, Evans CT, Miskevics S, Parada JP, Priebe M, Weaver FM . Long-term outcomes from nosocomial infections in persons with spinal cord injuries and disorders. Am J Infect Control 2007; 35: 393–400.
NCCLS. Performance Standards For Antimicrobial Susceptibility Testing. 2000; 7 th edn Approved standard M2-A7.
Tenover FC, Raney PM, Williams PP, Rasheed JK, Biddle JW, Oliver A et al. Evaluation of the NCCLS extended-spectrum β-lactamase confirmation methods for Escherichia coli with isolates collected during Project ICARE. J Clin Microbiol 2003; 41: 3142–3146.
Lillie PJ, Allen J, Hall C, Walsh C, Adams K, Thaker H et al. Long-term mortality following bloodstream infection. Clin Microbiol Infect 2013; 19: 955–960.
Rahimkhani M, Mordadi A, Varmazyar S, Tavakoli A . Evaluation of urinary interleukin-8 levels in patients with spinal cord injury. Recent Pat Antiinfect Drug Discov 2015; 9: 144–149.
Kreger BE, Craven DE, McCabe WR . Gram-negative bacteremia. Re-evaluation of clinical features and treatment in 612 patients. Am J Med 1980; 68: 344–355.
Kreger BE, Craven DE, Carling PC, McCabe WR . Gram-negative bacteremia. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med 1980; 68: 332–343.
Rahimkhani M, Nikfallah A, Saberian M, Mordadi A, Varmazyar S, Tavakoli A . Urinary tract infection in spinal cord injuries. Asian J Pharm Clin Res 2014; 7: 178–182.
World Health Organization. Antimicrobial resistance. 2015 Available at http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 1 July 2015)..
Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA . Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch Phys Med Rehabil 2000; 81: 764–769.
Darouiche RO, Hull RA . Bacterial interference for prevention of urinary tract infection. Clin Infect Dis 2012; 55: 1400–1407.
Mylotte JM, Graham R, Kahler L, Young L, Goodnough S . Epidemiology of nosocomial infection and resistant organisms in patients admitted for the first time to an acute rehabilitation unit. Clin Infect Dis 2000; 30: 425–432.
Tacconelli E . New strategies to identify patients harbouring antibiotic-resistant bacteria at hospital admission. Clin Microbiol Infect 2006; 12: 102–109.
Peleg AY, Hooper DC . Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med 2010; 362: 1804–1813.
Vogelaers D, De Bels D, Foret F, Cran S, Gilbert E, Schoonheydt K et al. Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates-a multicentre, observational survey in critically ill patients. Int J Antimicrob Agents 2010; 35: 375–381.
Lim CJ, Cheng AC, Kennon J, Spelman D, Hale D, Melican G et al. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case-control study. J Antimicrob Chemother 2014; 69: 1972–1980.
Falagas ME, Kopterides P . Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J Hosp Infect 2006; 64: 7–15.
Cordery RJ, Roberts CH, Cooper SJ, Bellinghan G, Shetty N . Evaluation of risk factors for the acquisition of bloodstream infections with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in the intensive care unit; antibiotic management and clinical outcome. J Hosp Infect 2008; 68: 108–115.
Wenzel RP, Edmond MB . The impact of hospital-acquired bloodstream infections. Emerg Infect Dis 2001; 7: 174–177.
Weinstein MP, Murphy JR, Reller LB, Lichtenstein KA . The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis 1983; 5: 54–70.
Guo B, Abdelraouf K, Ledesma KR, Nikolaou M, Tam VH . Predicting bacterial fitness cost associated with drug resistance. J Antimicrob Chemother 2012; 67: 928–932.
Luciani F, Sisson SA, Jiang H, Francis AR, Tanaka MM . The epidemiological fitness cost of drug resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2009; 106: 14711–14715.
Martínez JL, Baquero F . Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev 2002; 15: 647–679.
Obritsch MD, Fish DN, MacLaren R, Jung R . Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy 2005; 25: 1353–1364.
Falagas ME, Karageorgopoulos DE . Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: need for international harmonization in terminology. Clin Infect Dis 2008; 46: 1121–1122.
Falagas ME, Koletsi PK, Bliziotis IA . The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol 2006; 55: 1619–1629.
Hachem RY, Chemaly RF, Ahmar CA, Jiang Y, Boktour MR, Rjaili GA et al. Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob Agents Chemother 2007; 51: 1905–1911.
Acknowledgements
We thank Elodie Choisy, Clara Duran and the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dinh, A., Saliba, M., Saadeh, D. et al. Blood stream infections due to multidrug-resistant organisms among spinal cord-injured patients, epidemiology over 16 years and associated risks: a comparative study. Spinal Cord 54, 720–725 (2016). https://doi.org/10.1038/sc.2015.234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.234
This article is cited by
-
Bloodstream infections in older population: epidemiology, outcome, and impact of multidrug resistance
European Journal of Clinical Microbiology & Infectious Diseases (2021)
-
Factors associated with bacteraemia due to multidrug-resistant organisms among bacteraemic patients with multidrug-resistant organism carriage: a case control study
Antimicrobial Resistance & Infection Control (2018)
-
Ceftolozane/tazobactam for febrile UTI due to multidrug-resistant Pseudomonas aeruginosa in a patient with neurogenic bladder
Spinal Cord Series and Cases (2017)
-
Outcome of bloodstream infections among spinal cord injury patients and impact of multidrug-resistant organisms
Spinal Cord (2017)