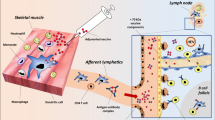
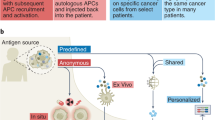
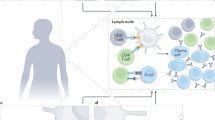
Abstract
From early excitement to total disillusionment and now gaining momentum again, cancer vaccination has been a real roller-coaster ride. Are the current expectations justified or will there be further disappointment? In this perspective article, I will discuss why the rationale of cancer vaccination does make sense after all and offer some thoughts and guidelines on how to get it right this time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nauts, H. C. Bacteria and cancer: antagonisms and benefits. Cancer Surv. 8, 713–723 (1989).
Prehn, R. T. & Main, J. M. Immunity to methylcholantrene-induced sarcomas. J. Natl Cancer Inst. 18, 769–778 (1957).
Klein, G., Sogren, H. O., Klein, H. & Hellstrom, K. E. Demonstration of resistance against methylcholantrene-induced sarcomas in primary autochtonous host. Cancer Res. 20, 1561–1572 (1960).
Thomas, L. Discussions of cellular and humoral aspects of the hypersensitive states (Hoeber–Harper, New York, 1959).
Burnet, F. M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13, 1–27 (1970).
Livingston, P. Active specific immunotherapy in the treatment of patients with cancer. Immunol. Allergy Clin. North Am. 11, 410–430 (1991).
Hewitt, H. B., Blake, E. R. & Walder, A. S. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br. J. Cancer 33, 241–259 (1976).
Stutman, O. Chemical carcinogenesis in nude mice: comparison between nude mice from homozygous matings and heterozygous matings and effect of age and carcinogen dose. J. Natl Cancer Inst. 62, 353–358 (1979).
Rygaard, J. & Povlsen, C. O. The mouse mutant nude does not develop spontaneous tumours. An argument against immunological surveillance. Acta Pathol. Microbiol. Scand. Microbiol. Immunol. [B] 82, 99–106 (1974).
Gatti, R. A. & Good, R. A. Occurrence of malignancy in immunodeficiency diseases. A literature review. Cancer 28, 89–98 (1971).
Penn, I. Tumors of the immunocompromised patient. Annu. Rev. Med. 39, 63–73 (1988).
Van Pel, A. & Boon, T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc. Natl Acad. Sci. USA 79, 4718–4722 (1982).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 3, 991–998 (2002).
Smyth, M. J., Godfrey, D. I. & Trapani, J. A. A fresh look at tumor immunosurveillance and immunotherapy. Nature Immunol. 2, 293–299 (2001).
Pardoll, D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21, 807–839 (2003).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Pawelec, G., Zeuthen, J. & Kiessling, R. Escape from host-antitumor immunity. Crit. Rev. Oncog. 8, 111–141 (1997).
Marincola, F. M., Jaffee, E. M., Hicklin, D. J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Dalgleish, A. G. Cancer vaccines. Br. J. Cancer 82, 1619–1624 (2000).
Mason, D. A very high level of crossreactivity is an essential feature of the T- cell receptor. Immunol. Today 19, 395–404 (1998).
Kersh, G. J. & Allen, P. M. Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands. J. Exp. Med. 184, 1259–1268 (1996).
Wucherpfennig, K. W. & Strominger, J. L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705 (1995).
Schild, H., Rotzschke, O., Kalbacher, H. & Rammensee, H. G. Limit of T cell tolerance to self proteins by peptide presentation. Science 247, 1587–1589 (1990).
Heiser, A. et al. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J. Immunol. 164, 5508–5514 (2000).
Bouneaud, C., Kourilsky, P. & Bousso, P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity 13, 829–840 (2000).
Morgan, D. J. et al. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 160, 643–651 (1998).
de Visser, K. E. et al. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur. J. Immunol. 30, 1458–1468 (2000).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Janeway, C. A. Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13, 11–16 (1992).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Reis e Sousa, C. Dendritic cells as sensors of infection. Immunity 14, 495–498 (2001).
Sebzda, E. et al. Selection of the T cell repertoire. Annu. Rev. Immunol. 17, 829–874 (1999).
Van Parijs, L. & Abbas, A. K. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science 280, 243–248 (1998).
Adler, A. J. et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 187, 1555–1564 (1998).
Kurts, C., Kosaka, H., Carbone, F. R., Miller, J. F. & Heath, W. R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186, 239–245 (1997).
Steinman, R. M. & Nussenzweig, M. C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl Acad. Sci. USA 99, 351–358 (2002).
Shevach, E. M. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18, 423–449 (2000).
Roncarolo, M. G., Bacchetta, R., Bordignon, C., Narula, S. & Levings, M. K. Type 1 T regulatory cells. Immunol. Rev. 182, 68–79 (2001).
Read, S. & Powrie, F. CD4+ regulatory T cells. Curr. Opin. Immunol. 13, 644–649 (2001).
Schirrmacher, V. et al. Immunization with virus-modified tumor cells. Semin. Oncol. 25, 677–696 (1998).
Chan, A. D. & Morton, D. L. Active immunotherapy with allogeneic tumor cell vaccines: present status. Semin. Oncol. 25, 611–622 (1998).
Welsh, R. M. & Selin, L. K. No one is naive: the significance of heterologous T-cell immunity. Nature Rev. Immunol. 2, 417–426 (2002).
Adams, A. B., Pearson, T. C. & Larsen, C. P. Heterologous immunity: an overlooked barrier to tolerance. Immunol. Rev. 196, 147–160 (2003).
Alexander-Miller, M. A., Leggatt, G. R. & Berzofsky, J. A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl Acad. Sci. USA 93, 4102–4107 (1996).
Zeh, H. J. 3rd, Perry-Lalley, D., Dudley, M. E., Rosenberg, S. A. & Yang, J. C. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 162, 989–994 (1999).
Cordaro, T. A., de Visser, K. E., Tirion, F. H., Schumacher, T. N. & Kruisbeek, A. M. Can the low-avidity self-specific T cell repertoire be exploited for tumor rejection? J. Immunol. 168, 651–660 (2002).
Gilboa, E. The makings of a tumor rejection antigen. Immunity 11, 263–270 (1999).
Loeb, L. A. A mutator phenotype in cancer. Cancer Res. 61, 3230–3239 (2001).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Zitvogel, L. et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J. Exp. Med. 183, 87–97 (1996).
Fields, R. C., Shimizu, K. & Mule, J. J. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc. Natl Acad. Sci. USA 95, 9482–9487 (1998).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184, 465–472 (1996).
Boczkowski, D., Nair, S. K., Nam, J. H., Lyerly, H. K. & Gilboa, E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 60, 1028–1034 (2000).
Grunebach, F., Muller, M. R., Nencioni, A. & Brossart, P. Delivery of tumor-derived RNA for the induction of cytotoxic T-lymphocytes. Gene Ther. 10, 367–374 (2003).
Nair, S. K. et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nature Med. 6, 1011–1017 (2000).
Vonderheide, R. H., Hahn, W. C., Schultze, J. L. & Nadler, L. M. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 10, 673–679 (1999).
Schmitz, M. et al. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 60, 4845–4849 (2000).
Andersen, M. H. et al. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 61, 5964–5968 (2001).
Zeis, M. et al. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-rna-transfected dendritic cells. J. Immunol. 170, 5391–5397 (2003).
Barsoum, A. L., Rohrer, J. W. & Coggin, J. H. 37kDa oncofetal antigen is an autoimmunogenic homologoue of the 37kDa laminin receptor precursor. Cell. Mol. Biol. Lett. 5, 207–230 (2000).
Biragyn, A. et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J. Immunol. 167, 6644–6653 (2001).
You, Z., Huang, X., Hester, J., Toh, H. C. & Chen, S. Y. Targeting dendritic cells to enhance DNA vaccine potency. Cancer Res. 61, 3704–3711 (2001).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor dec-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T Cell Tolerance. J. Exp. Med. 196, 1627–1638 (2002).
de Gruijl, T. D. et al. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J. Immunol. 169, 5322–5331 (2002).
Almand, B. et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 (2001).
Gabrilovich, D. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92, 4150–4166 (1998).
Fong, L. & Engleman, E. G. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 18, 245–273 (2000).
Gunzer, M. & Grabbe, S. Dendritic cells in cancer immunotherapy. Crit. Rev. Immunol. 21, 133–145 (2001).
Brossart, P., Wirths, S., Brugger, W. & Kanz, L. Dendritic cells in cancer vaccines. Exp. Hematol. 29, 1247–1255 (2001).
Pardoll, D. M. & Topalian, S. L. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 10, 588–594 (1998).
Toes, R. E., Ossendorp, F., Offringa, R. & Melief, C. J. CD4 T cells and their role in antitumor immune responses. J. Exp. Med. 189, 753–756 (1999).
Wang, R. F. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 22, 269–276 (2001).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Sun, J. C. & Bevan, M. J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Shedlock, D. J. & Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300, 337–339 (2003).
Kalams, S. A. & Walker, B. D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188, 2199–2204 (1998).
Zajac, A. J., Murali-Krishna, K., Blattman, J. N. & Ahmed, R. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr. Opin. Immunol. 10, 444–449 (1998).
Kaplan, D. H. et al. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Qin, Z. & Blankenstein, T. CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN γ receptor expression by nonhematopoietic cells. Immunity 12, 677–686 (2000).
Mumberg, D. et al. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl Acad. Sci. USA 96, 8633–8638 (1999).
Greenberg, P. D., Kern, D. E. & Cheever, M. A. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J. Exp. Med. 161, 1122–1134 (1985).
Hock, H., Dorsch, M., Diamantstein, T. & Blankenstein, T. Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J. Exp. Med. 174, 1291–1298 (1991).
Levitsky, H. I., Lazenby, A., Hayashi, R. J. & Pardoll, D. M. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J. Exp. Med. 179, 1215–1224 (1994).
Hung, K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Marrack, P. et al. T-cell survival. Immunol. Rev. 165, 279–285 (1998).
Sprent, J., Zhang, X., Sun, S. & Tough, D. T-cell turnover in vivo and the role of cytokines. Immunol. Lett. 65, 21–25 (1999).
Hendriks, J. et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nature Immunol. 1, 433–440 (2000).
Weinberg, A. D., Vella, A. T. & Croft, M. OX-40: life beyond the effector T cell stage. Semin. Immunol. 10, 471–480 (1998).
Kwon, B., Lee, H. W. & Kwon, B. S. New insights into the role of 4-1BB in immune responses: beyond CD8+ T cells. Trends Immunol. 23, 378–380 (2002).
Sullenger, B. A. & Gilboa, E. Emerging clinical applications of RNA. Nature 418, 252–258 (2002).
Alegre, M. L., Frauwirth, K. A. & Thompson, C. B. T-cell regulation by CD28 and CTLA-4. Nature Rev. Immunol. 1, 220–228 (2001).
Salomon, B. & Bluestone, J. A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19, 225–252 (2001).
Chambers, C. A., Kuhns, M. S., Egen, J. G. & Allison, J. P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19, 565–594 (2001).
Carreno, B. M. & Collins, M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 20, 29–53 (2002).
Egen, J. G., Kuhns, M. S. & Allison, J. P. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Immunol. 3, 611–618 (2002).
Bass, K. K. & Mastrangelo, M. J. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol. Immunother. 47, 1–12 (1998).
Shimizu, J., Yamazaki, S. & Sakaguchi, S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163, 5211–5218 (1999).
Onizuka, S. et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 59, 3128–3133 (1999).
Sutmuller, R. P. et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194, 823–832 (2001).
Gorelik, L. & Flavell, R. A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nature Med. 7, 1118–1122 (2001).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Med. 8, 793–800 (2002).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Kusmartsev, S. & Gabrilovich, D. I. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol. Immunother. 51, 293–298 (2002).
Bronte, V., Serafini, P., Mazzoni, A., Segal, D. M. & Zanovello, P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 24, 302–306 (2003).
Kusmartsev, S. et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63, 4441–4449 (2003).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Hodi, F. S. et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl Acad. Sci. USA 100, 4712–4717 (2003).
Surh, C. D. & Sprent, J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192, F9–F14 (2000).
Ge, Q., Rao, V. P., Cho, B. K., Eisen, H. N. & Chen, J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc. Natl Acad. Sci. USA 98, 1728–1733 (2001).
Kassiotis, G., Zamoyska, R. & Stockinger, B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J. Exp. Med. 197, 1007–1016 (2003).
Borrello, I. et al. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood 95, 3011–3019 (2000).
Dummer, W. et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 110, 185–192 (2002).
Hu, H. M., Poehlein, C. H., Urba, W. J. & Fox, B. A. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 62, 3914–3919 (2002).
Asavaroengchai, W., Kotera, Y. & Mule, J. J. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc. Natl Acad. Sci. USA 99, 931–936 (2002).
Naftzger, C. et al. Immune response to a differentiation antigen induced by altered antigen: a study of tumor rejection and autoimmunity. Proc. Natl Acad. Sci. USA 93, 14809–14814 (1996).
Overwijk, W. W. et al. Vaccination with a recombinant vaccinia virus encoding a 'self' antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc. Natl Acad. Sci. USA 96, 2982–2987 (1999).
Fong, L., Ruegg, C. L., Brockstedt, D., Engleman, E. G. & Laus, R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J. Immunol. 159, 3113–3117 (1997).
Dittel, B. N., Visintin, I., Merchant, R. M. & Janeway, C. A. Jr. Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J. Immunol. 163, 32–39 (1999).
Ludewig, B. et al. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J. Exp. Med. 191, 795–804 (2000).
Bronte, V. et al. Genetic vaccination with 'self' tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res. 60, 253–258 (2000).
Melero, I. et al. Immunological ignorance of an E7-encoded cytolytic T-lymphocyte epitope in transgenic mice expressing the E7 and E6 oncogenes of human papillomavirus type 16. J. Virol. 71, 3998–4004 (1997).
Hu, J. et al. An evaluation of the potential to use tumor-associated antigens as targets for antitumor T cell therapy using transgenic mice expressing a retroviral tumor antigen in normal lymphoid tissues. J. Exp. Med. 177, 1681–1690 (1993).
Maeurer, M. J. et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J. Clin. Invest. 98, 1633–1641 (1996).
Jager, E. et al. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int. J. Cancer 71, 142–147 (1997).
Yee, C. et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA 99, 16168–16173 (2002).
Yewdell, J. W. & Bennink, J. R. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17, 51–88 (1999).
Albert, M. L. & Darnell, R. B. Paraneoplastic neurological degenerations: keys to tumour immunity. Nature Rev. Cancer 4, 36–44 (2004).
Roth, J. et al. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc. Natl Acad. Sci. USA 93, 4781–4786 (1996).
Speiser, D. E. et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 186, 645–653 (1997).
Van den Eynde, B. J. & Morel, S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Curr. Opin. Immunol. 13, 147–153 (2001).
Diefenbach, A., Jensen, E. R., Jamieson, A. M. & Raulet, D. H. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413, 165–171 (2001).
Groh, V. et al. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nature Immunol. 2, 255–260 (2001).
Dvorak, H. F., Nagy, J. A. & Dvorak, A. M. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cells 3, 77–85 (1991).
Simon, R. M. et al. Clinical trial designs for the early clinical development of therapeutic cancer vaccines. J. Clin. Oncol. 19, 1848–1854 (2001).
Nair, S. et al. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J. Immunol. 171, 6275–6282 (2003).
Santulli-Marotto, S., Nair, S. K., Rusconi, C., Sullenger, B. & Gilboa, E. Multivalent RNA aptamers that inhibit ctla-4 and enhance tumor immunity. Cancer Res. 63, 7483–7489 (2003).
Nair, S. et al. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood 102, 964–971 (2003).
Zhao, Y., Boczkowski, D., Nair, S. K. & Gilboa, E. Inhibition of invariant chain expression in dendritic cells presenting endogenous antigens stimulates CD4+ T-cell responses and tumor immunity. Blood 102, 4137–4142 (2003).
Liotta, L. A. & Kohn, E. C. The microenvironment of the tumour-host interface. Nature 411, 375–379 (2001).
Wei, Y. Q. et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nature Med. 6, 1160–1166 (2000).
Li, Y. et al. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J. Exp. Med. 195, 1575–1584 (2002).
Niethammer, A. G. et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nature Med. 8, 1369–1375 (2002).
Kerbel, R. S. A cancer therapy resistant to resistance. Nature 390, 335–336 (1997).
Cherrington, J. M., Strawn, L. M. & Shawver, L. K. New paradigms for the treatment of cancer: the role of anti-angiogenesis agents. Adv. Cancer Res. 79, 1–38 (2000).
Nelson, A. R., Fingleton, B., Rothenberg, M. L. & Matrisian, L. M. Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 18, 1135–1149 (2000).
Kerbel, R. S. et al. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev. 20, 79–86 (2001).
Acknowledgements
Correction: The DOI number given for this article in the May 2004 print issue of Nature Reviews Cancer was wrong. The correct DOI number is: doi:10.1038/nrc1359.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author is the scientific founder and owns stock in Merix Bioscience, a cancer immunotherapy company.
Rights and permissions
About this article
Cite this article
Gilboa, E. The promise of cancer vaccines. Nat Rev Cancer 4, 401–411 (2004). https://doi.org/10.1038/nrc1359
Issue Date:
DOI: https://doi.org/10.1038/nrc1359
This article is cited by
-
Quality assessment on the long-term cryopreservation and nucleic acids extraction processes implemented in the andalusian public biobank
Cell and Tissue Banking (2019)
-
The expression of Foxp3 and TLR4 in cervical cancer: association with immune escape and clinical pathology
Archives of Gynecology and Obstetrics (2017)
-
Generation of cytotoxic T lymphocytes specific for native or modified peptides derived from the epidermal growth factor receptor pathway substrate 8 antigen
Cancer Immunology, Immunotherapy (2015)
-
Immunotherapy of tumor with vaccine based on basic fibroblast growth factor-activated fibroblasts
Journal of Cancer Research and Clinical Oncology (2014)