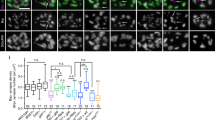
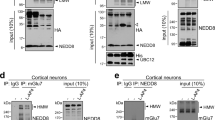
Abstract
Formation of appropriate synaptic connections is critical for proper functioning of the brain. After initial synaptic differentiation, active synapses are stabilized by neural activity-dependent signals to establish functional synaptic connections. However, the molecular mechanisms underlying activity-dependent synapse maturation remain to be elucidated. Here we show that activity-dependent ectodomain shedding of signal regulatory protein-α (SIRPα) mediates presynaptic maturation. Two target-derived molecules, fibroblast growth factor 22 and SIRPα, sequentially organize the glutamatergic presynaptic terminals during the initial synaptic differentiation and synapse maturation stages, respectively, in the mouse hippocampus. SIRPα drives presynaptic maturation in an activity-dependent fashion. Remarkably, neural activity cleaves the extracellular domain of SIRPα, and the shed ectodomain in turn promotes the maturation of the presynaptic terminal. This process involves calcium/calmodulin-dependent protein kinase, matrix metalloproteinases and the presynaptic receptor CD47. Finally, SIRPα-dependent synapse maturation has an impact on synaptic function and plasticity. Thus, ectodomain shedding of SIRPα is an activity-dependent trans-synaptic mechanism for the maturation of functional synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lipska, B.K., Halim, N.D., Segal, P.N. & Weinberger, D.R. Effects of reversible inactivation of the neonatal ventral hippocampus on behavior in the adult rat. J. Neurosci. 22, 2835–2842 (2002).
Pfeiffer, B.E. et al. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron 66, 191–197 (2010).
Kasai, H., Fukuda, M., Watanabe, S., Hayashi-Takagi, A. & Noguchi, J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33, 121–129 (2010).
Sanes, J.R. & Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Waites, C.L., Craig, A.M. & Garner, C.C. Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci. 28, 251–274 (2005).
Fox, M.A. & Umemori, H. Seeking long-term relationship: axon and target communicate to organize presynaptic differentiation. J. Neurochem. 97, 1215–1231 (2006).
Dalva, M.B., McClelland, A.C. & Kayser, M.S. Cell adhesion molecules: signaling functions at the synapse. Nat. Rev. Neurosci. 8, 206–220 (2007).
Goda, Y. & Davis, G.W. Mechanisms of synapse assembly and disassembly. Neuron 40, 243–264 (2003).
Tessier, C.R. & Broadie, K. Activity-dependent modulation of neural circuit synaptic connectivity. Front. Mol. Neurosci. 2, 8 (2009).
Kano, M. & Hashimoto, K. Synapse elimination in the central nervous system. Curr. Opin. Neurobiol. 19, 154–161 (2009).
Zhang, L.I. & Poo, M. Electrical activity and development of neural circuits. Nat. Neurosci. 4, 1207–1214 (2001).
Bleckert, A. & Wong, R.O. Identifying roles for neurotransmission in circuit assembly: insights gained from multiple model systems and experimental approaches. Bioessays 33, 61–72 (2011).
Kay, L., Humphreys, L., Eickholt, B.J. & Burrone, J. Neuronal activity drives matching of pre- and postsynaptic function during synapse maturation. Nat. Neurosci. 14, 688–690 (2011).
Flavell, S.W. & Greenberg, M.E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 31, 563–590 (2008).
Umemori, H., Linhoff, M.W., Onitz, D.M. & Sanes, J.R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270 (2004).
Umemori, H. & Sanes, J.R. Signal regulatory proteins (SIRPs) are secreted presynaptic organizing molecules. J. Biol. Chem. 283, 34053–34061 (2008).
Terauchi, A. et al. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 465, 783–787 (2010).
van Beek, E.M., Cochrane, F., Barclay, A.N. & van den Berg, T.K. Signal regulatory proteins in the immune system. J. Immunol. 175, 7781–7787 (2005).
Barclay, A.N. & Brown, M.H. The SIRP family of receptors and immune regulation. Nat. Rev. Immunol. 6, 457–464 (2006).
Matozaki, T., Murata, Y., Okazawa, H. & Ohnishi, H. Functions and molecular mechanisms of the CD47-SIRPα signaling pathway. Trends Cell Biol. 19, 72–80 (2009).
Danglot, L., Triller, A. & Marty, S. The development of hippocampal interneurons in rodents. Hippocampus 16, 1032–1060 (2006).
Steward, O. & Falk, P.M. Selective localization of polyribosomes beneath developing synapses: a quantitative analysis of the relationships between polyribosomes and developing synapses in the hippocampus and dentate gyrus. J. Comp. Neurol. 314, 545–557 (1991).
Bouvier, D. et al. Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J. Neurochem. 106, 682–695 (2008).
Biederer, T. & Scheiffele, P. Mixed-culture assays for analyzing neuronal synapse formation. Nat. Protoc. 2, 670–676 (2007).
Guo, C., Yang, W. & Lobe, C.G. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 32, 8–18 (2002).
Yasuda, M. et al. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron 70, 1128–1142 (2011).
Wayman, G.A., Lee, Y.S., Tokumitsu, H., Silva, A.J. & Soderling, T.R. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59, 914–931 (2008).
Ethell, I.M. & Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J. Neurosci. Res. 85, 2813–2823 (2007).
Lindberg, F.P. et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274, 795–798 (1996).
Chang, H.P., Lindberg, F.P., Wang, H.L., Huang, A.M. & Lee, E.H. Impaired memory retention and decreased long-term potentiation in integrin-associated protein-deficient mice. Learn. Mem. 6, 448–457 (1999).
Shen, K. & Cowan, C.W. Guidance molecules in synapse formation and plasticity. Cold Spring Harb. Perspect. Biol. 2, a001842 (2010).
Williams, M.E., de Wit, J. & Ghosh, A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron 68, 9–18 (2010).
Siddiqui, T.J. & Craig, A.M. Synaptic organizing complexes. Curr. Opin. Neurobiol. 21, 132–143 (2011).
Wang, X.X. & Pfenninger, K.H. Functional analysis of SIRPα in the growth cone. J. Cell Sci. 119, 172–183 (2006).
Ohnishi, H. et al. Stress-evoked tyrosine phosphorylation of signal regulatory protein α regulates behavioral immobility in the forced swim test. J. Neurosci. 30, 10472–10483 (2010).
Hatherley, D. et al. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol. Cell 31, 266–277 (2008).
Hatherley, D., Graham, S.C., Harlos, K., Stuart, D.I. & Barclay, A.N. Structure of signal-regulatory protein α: a link to antigen receptor evolution. J. Biol. Chem. 284, 26613–26619 (2009).
Edwards, D.R., Handsley, M.M. & Pennington, C.J. The ADAM metalloproteinases. Mol. Aspects Med. 29, 258–289 (2008).
Reiss, K. & Saftig, P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin. Cell Dev. Biol. 20, 126–137 (2009).
Bai, G. & Pfaff, S.L. Protease regulation: the yin and yang of neural development and disease. Neuron 72, 9–21 (2011).
Peixoto, R.T. et al. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron 76, 396–409 (2012).
Suzuki, K. et al. Activity-dependent proteolytic cleavage of neuroligin-1. Neuron 76, 410–422 (2012).
Schaeren-Wiemers, N. & Gerfin-Moser, A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431–440 (1993).
Uesaka, T. et al. Conditional ablation of GFRα1 in postmigratory enteric neurons triggers unconventional neuronal death in the colon and causes a Hirschsprung's disease phenotype. Development 134, 2171–2181 (2007).
Woodhams, P.L., Webb, M., Atkinson, D.J. & Seeley, P.J. A monoclonal antibody, Py, distinguishes different classes of hippocampal neurons. J. Neurosci. 9, 2170–2181 (1989).
Oldenborg, P.A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Ohnishi, H. et al. Ectodomain shedding of SHPS-1 and its role in regulation of cell migration. J. Biol. Chem. 279, 27878–27887 (2004).
Hahn, C.G. et al. The post-synaptic density of human postmortem brain tissues: an experimental study paradigm for neuropsychiatric illnesses. PLoS ONE 4, e5251 (2009).
Fox, M.A. & Sanes, J.R. Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 503, 280–296 (2007).
Acknowledgements
We thank J. Sanes for critical comments on the manuscript; H. Enomoto for pSV loxP sv40 intron polyA EGFP FRTneo plasmid; A. Murayama and L. Kee for plasmid construction; E. Gibbs for help with in situ hybridization; D. Sorenson for help with electron microscopy; M. Zhang, R. Carson and A. Williams for technical assistance; and E. Hughes, Y. Qu, K. Childs, G. Gavrilina, D. Vanheyningen and the Transgenic Animal Model Core of the University of Michigan for preparation of SIRPα knockout mice. Core support was provided by the University of Michigan Center for Organogenesis. This work was supported by the Ester A. & Joseph Klingenstein Fund, the Edward Mallinckrodt Jr. Foundation, the March of Dimes Foundation, the Whitehall Foundation and US National Institutes of Health grants MH091429, NS070005 and MH092614 (H.U.).
Author information
Authors and Affiliations
Contributions
H.U. designed experiments and prepared the manuscript. A.B.T., A.T., L.Y.Z., E.M.J.-V. and D.J.L. performed experiments. M.A.S. and H.U. supervised the project. All authors analyzed data and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 5990 kb)
Rights and permissions
About this article
Cite this article
Toth, A., Terauchi, A., Zhang, L. et al. Synapse maturation by activity-dependent ectodomain shedding of SIRPα. Nat Neurosci 16, 1417–1425 (2013). https://doi.org/10.1038/nn.3516
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3516
This article is cited by
-
Microglia as hackers of the matrix: sculpting synapses and the extracellular space
Cellular & Molecular Immunology (2021)
-
Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration
Nature Communications (2021)
-
Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders
Nature Reviews Immunology (2021)
-
Microglial immune checkpoint mechanisms
Nature Neuroscience (2018)
-
SheddomeDB: the ectodomain shedding database for membrane-bound shed markers
BMC Bioinformatics (2017)