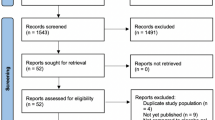
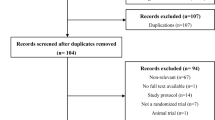
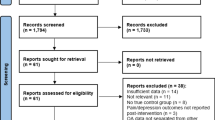
Abstract
Nonpharmacologic treatments (including surgery, technical operation, rehabilitation, physiotherapy, education or the use of external technical devices) represent a wide range of treatments for chronic rheumatic disease. Randomized, controlled trials (RCTs) are recognized as the best method for avoiding bias in assessing nonpharmacologic treatments. Designs such as nonrandomized studies, cluster-randomized trials, patient-preference trials, modified Zelen-design trials, tracker trials and expertise-based RCTs could, however, be used to assess such treatments. Assessing nonpharmacologic treatments involves methodologic issues linked to difficulties associated with blinding, duration of the study, main outcomes of the study, difficulties associated with standardizing the intervention and the influence of health-care providers. Hence, these treatments cannot be assessed according to the standards used for pharmacologic treatments. As well, specific instruments such as A CheckList to Evaluate A Report of a NonPharmacological Trial (CLEAR NPT) should be used to assess the quality of reports in this field. Important reporting guidelines that take an evidence-based approach to improve the quality of reports from RCTs, such as the Consolidated Standards of Reporting Trials (CONSORT) statement, should be extended to take these issues into account.
Key Points
-
The planning and reporting of randomized, controlled trials assessing nonpharmacologic treatments should take into account certain difficulties
-
Such difficulties are those associated with blinding, and the influence of health-care providers and the centers' number of procedures on the success of the treatment
-
A clear definition of all the components of the intervention is particularly important
-
Specific designs such as cluster-randomized trials, patient-preference trials, modified Zelen-design trials, tracker trials and expertise-based, randomized trials are also available for assessing these treatments
-
Efforts are required to enhance the assessment and reporting of harm associated with nonpharmacologic treatments
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pincus T (2002) Limitations of randomized clinical trials in chronic diseases: explanations and recommendations. Adv Mind Body Med 18: 14–21
Hill CL et al. (2002) Secular changes in the quality of published randomized clinical trials in rheumatology. Arthritis Rheum 46: 779–784
Boutron I et al. (2003) Methodological differences in clinical trials evaluating nonpharmacological and pharmacological treatments of hip and knee osteoarthritis. JAMA 290: 1062–1070
Boutron I et al. (2004) Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J Clin Epidemiol 57: 543–550
Abel U and Koch A (1999) The role of randomization in clinical studies: myths and beliefs. J Clin Epidemiol 52: 487–497
Ioannidis JP et al. (2001) Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 286: 821–830
Lauritzen JB et al. (1993) Effect of external hip protectors on hip fractures. Lancet 341: 11–13
Puffer S et al. (2003) Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ 327: 785–789
Donner A and Klar N (2000) Design and analysis of cluster randomization trials in health research. London: Arnold Publishing Co.
King M et al. (2005) Impact of participant and physician intervention preferences on randomized trials: a systematic review. JAMA 293: 1089–1099
Torgerson D and Moffett JK (2005) Patient preference and validity of randomized controlled trials. JAMA 294: 41–42
Torgerson DJ and Sibbald B (1998) Understanding controlled trials. What is a patient preference trial? BMJ 316: 360
McPherson K et al. (1997) Are randomized controlled trials controlled? Patient preferences and unblind trials. J R Soc Med 90: 652–656
Campbell R et al. (2005) Adapting the randomized consent (Zelen) design for trials of behavioural interventions for chronic disease: feasibility study. J Health Serv Res Policy 10: 220–225
Torgerson DJ and Roland M (1998) What is Zelen's design? BMJ 316: 606
Zelen M (1992) Randomised consent trials. Lancet 340: 375
Altman DG et al. (1995) Randomised consent designs in cancer clinical trials. Eur J Cancer 31A: 1934–1944
Lilford RJ et al. (2000) Trials and fast changing technologies: the case for tracker studies. BMJ 320: 43–46
Devereaux PJ et al. (2005) Need for expertise based randomised controlled trials. BMJ 330: 88
Lilford R et al. (2004) Trials in surgery. Br J Surg 91: 6–16
Schulz KF et al. (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273: 408–412
Pildal J et al. (2005) Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. BMJ 330: 1049
Noseworthy JH et al. (1994) The impact of blinding on the results of a randomized, placebo- controlled multiple sclerosis clinical trial. Neurology 44: 16–20
Juni P et al. (2001) Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 323: 42–46
Moffett JK et al. (1999) Randomised controlled trial of exercise for low back pain: clinical outcomes, costs, and preferences. BMJ 319: 279–283
Moseley JB et al. (2002) A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 347: 81–88
Bradley JD et al. (2002) Tidal irrigation as treatment for knee osteoarthritis: a sham- controlled, randomized, double-blinded evaluation. Arthritis Rheum 46: 100–108
Ravaud P et al. (1999) Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arthritis Rheum 42: 475–482
Streitberger K and Kleinhenz J (1998) Introducing a placebo needle into acupuncture research. Lancet 352: 364–365
Paterson C and Dieppe P (2005) Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ 330: 1202–1205
Spigt MG et al. (2005) The validity and ethics of giving placebo in a randomized nonpharmacologic trial was evaluated. J Clin Epidemiol 58: 350–356
Hróbjartsson A and Gøtzsche PC (2004) Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med 256: 91–100
Auleley GR et al. (2004) The methods for handling missing data in clinical trials influence sample size requirements. J Clin Epidemiol 57: 447–453
Baron G et al. (2005) Violation of the intent-to-treat principle and rate of missing data in superiority trials assessing structural outcomes in rheumatic diseases. Arthritis Rheum 52: 1858–1865
Molenberghs G et al. (2004) Analyzing incomplete longitudinal clinical trial data. Biostatistics 5: 445–464
van der Heijde D et al. (2005) Presentation and analysis of data on radiographic outcome in clinical trials: experience from the TEMPO study. Arthritis Rheum 52: 49–60
Campbell M et al. (2000) Framework for design and evaluation of complex interventions to improve health. BMJ 321: 694–696
Devereaux PJ et al. (2005) How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 331: 313–321
Herbert RD and Bo K (2005) Analysis of quality of interventions in systematic reviews. BMJ 331: 507–509
Halm EA et al. (2002) Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 137: 511–520
Begg CB et al. (2002) Variations in morbidity after radical prostatectomy. N Engl J Med 346: 1138–1144
Harmon JW et al. (1999) Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 230: 404–411
Rothwell PM and Warlow CP (1999) Interpretation of operative risks of individual surgeons. European Carotid Surgery Trialists' Collaborative Group. Lancet 353: 1325
Urbach DR and Baxter NN (2004) Does it matter what a hospital is “high volume” for? Specificity of hospital volume-outcome associations for surgical procedures: analysis of administrative data. Qual Saf Health Care 13: 379–383
Ramsay CR et al. (2000) Assessment of the learning curve in health technologies. A systematic review. Int J Technol Assess Health Care 16: 1095–1108
Cherkin DC et al. (2003) A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Ann Intern Med 138: 898–906
Ethgen M et al. (2005) Reporting of harm in randomized, controlled trials of nonpharmacologic treatment for rheumatic disease. Ann Intern Med 143: 20–25
Ernst E (2001) Prospective investigations into the safety of spinal manipulation. J Pain Symptom Manage 21: 238–242
Norheim AJ and Fonnebo V (1995) Adverse effects of acupuncture. Lancet 345: 1576
Lee KP et al. (1995) Neurologic complications following chiropractic manipulation: a survey of California neurologists. Neurology 45: 1213–1215
Moher D et al. (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354: 1896–1900
Juni P et al. (1999) The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282: 1054–1060
Moher D et al. (1995) Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials 16: 62–73
Katrak P et al. (2004) A systematic review of the content of critical appraisal tools. BMC Med Res Methodol 4: 22
Jadad AR et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12
Verhagen AP et al. (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51: 1235–1241
Khuri SF et al. (1999) Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg 230: 414–429
Soljak M (2002) Volume of procedures and outcome of treatment. BMJ 325: 787–788
Boutron I et al.: Assessing the quality of randomized controlled trials evaluating nonpharmacological treatments: a checklist to evaluate a report of nonpharmacological treatment (CLEAR NPT). J Clin Epidemiol, in press
Boutron I et al. (2005) A checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT) was developed using consensus. J Clin Epidemiol 58: 1233–1240
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ravaud, P., Boutron, I. Primer: assessing the efficacy and safety of nonpharmacologic treatments for chronic rheumatic diseases. Nat Rev Rheumatol 2, 313–319 (2006). https://doi.org/10.1038/ncprheum0194
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0194
This article is cited by
-
Intensive spa and exercise therapy program for returning to work for low back pain patients: a randomized controlled trial
Scientific Reports (2017)
-
Evaluation of the efficacy of a short-course, personalized self-management and intensive spa therapy intervention as active prevention of musculoskeletal disorders of the upper extremities (Muska): a research protocol for a randomized controlled trial
BMC Musculoskeletal Disorders (2016)
-
Évaluation des traitements non médicamenteux dans la maladie d’Alzheimer
Les cahiers de l'année gérontologique (2013)