Abstract
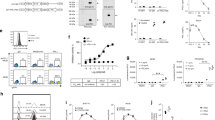
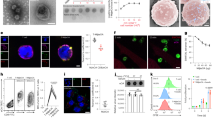
Immune responses to tumor-associated antigens are often dampened by a tumor-induced state of immune anergy. Previous work has attempted to overcome tumor-induced T-cell anergy by the direct injection of vectors carrying the genes encoding one of a variety of cytokines. We hypothesised that the polyclonal stimulation of T cells, preferably through the TCR complex, would result in a cascade of cytokines associated with T-cell activation and would be best able to overcome T-cell anergy. Here we use the highly attenuated MVA poxvirus to express on tumor cells, in vitro and in vivo, either of three membrane-bound monoclonal antibodies specific for murine TCR complex. Using this system, we have expressed antibodies specific for the CD3ɛ chain (KT3), TCRα/β complex (H57-597), and Vβ7 chain (TR310). Tumor cells bristling with these antibodies are capable of inducing murine T-cell proliferation and cytokine production. When injected into growing tumors (P815, RenCa, and B16F10), these constructs induce the activation of immune effector cells and result in the rejection of the tumor. Histological and FACS analysis of tumor-infiltrating leukocytes reveal that the injection of recombinant virus-expressing antibodies specific for the TCR complex attracts and activates (CD25+, CD69+) CD4 and CD8 lymphocytes. This approach represents a novel strategy to overcome T-cell anergy in tumors and allow the stimulation of tumor-specific T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weijtens ME, Willemsen RA, Valerio D, Stam K, Bolhuis RL . Single chain Ig/gamma gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity J Immunol 1996 157: 836–843
Tibben JG et al. Pharmacokinetics, biodistribution and biological effects of intravenously administered bispecific monoclonal antibody OC/TR F(ab′)2 in ovarian carcinoma patients Int J Cancer 1996 66: 477–483
Chattopadhyay U . Tumour immunotherapy: developments and strategies Immunol Today 1999 20: 480–482
Nanni P, Forni G, Lollini PL . Cytokine gene therapy: hopes and pitfalls Ann Oncol 1999 10: 261–266
Meuer SC et al. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones J Exp Med 1983 158: 988–993
Ellenhorn JD, Hirsch R, Schreiber H, Bluestone JA . In vivo administration of anti-CD3 prevents malignant progressor tumor growth Science 1988 242: 569–571
Liao KW, Lo YC, Roffler SR . Activation of lymphocytes by anti–CD3 single-chain antibody dimers expressed on the plasma membrane of tumor cells Gene Ther 2000 7: 339–347
de Ines C, Cochlovius B, Schmidt S, Kipriyanov S, Rode HJ, Little M . Apoptosis of a human melanoma cell line specifically induced by membrane-bound single-chain antibodies JImmunol 1999 163: 3948–3956
Paul S, Snary D, Hoebeke J et al. Redirection of macrophage cytotoxicity using a non-replicative live vector expressing a tumor-specific scFv Hum Gene Ther 2000 11: 1417–1428
Xie YC, Hwang C, Overwijk W et al. Induction of tumor antigen-specific immunity in vivo by a novel vaccinia vector encoding safety-modified simian virus 40 T antigen J Natl Cancer Inst 1999 91: 169–175
Venkatesan S, Baroudy BM, Moss B . Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene Cell 1981 25: 805–813
Chen L . Overcoming T cell ignorance by providing costimulation. Implications for the immune response against cancer Adv Exp Med Biol 1998 451: 159–165
Anilionis A, Wunner WH, Curtis PJ . Structure of the glycoprotein gene in rabies virus Nature 1981 294: 275–278
Mendiratta SK et al. Intratumoral delivery of IL-12 gene by polyvinyl polymeric vector system to murine renal and colon carcinoma results in potent antitumor immunity Gene Ther 1999 6: 833–839
Tomonari K . A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex Immunogenetics 1988 28: 455–458
Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M . Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors J Immunol 1989 142: 2736–2742
Okada CY, Holzmann B, Guidos C, Palmer E, Weissman IL . Characterization of a rat monoclonal antibody specific for a determinant encoded by the V beta 7 gene segment. Depletion of V beta 7+ T cells in mice with Mls-1a haplotype JImmunol 1990 144: 3473–3477
Paul S et al. Redirected cellular cytotoxicity by infection of effector cells with a recombinant vaccinia virus encoding a tumor-specific monoclonal antibody Cancer Gene Ther 2000 7: 615–623
Gajewski TF, Schell SR, Nau G, Fitch FW . Regulation of T-cell activation: differences among T-cell subsets Immunol Rev 1989 111: 79–110
Krutmann J, Kirnbauer R, Kock A et al. Cross-linking Fc receptors on monocytes triggers IL-6 production. Role in anti–CD3-induced T cell activation J Immunol 1990 145: 1337–1342
Zanders ED, Lamb JR, Feldmann M, Green N, Beverley PC . Tolerance of T-cell clones is associated with membrane antigen changes Nature 1983 303: 625–627
Norman DJ et al. A US clinical study of Orthoclone OKT3 in renal transplantation Transplant Proc 1987 19: 21–27
Henrickson M, Reid J, Bellet JS, Sawchuk SS, Hirsch R . Comparison of in vivo efficacy and mechanism of action of antimurine monoclonal antibodies directed against TCR alpha beta (H57-597) and CD3 (145-2C11) Transplantation 1995 60: 828–835
Weijtens ME, Hart EH, Bolhuis RL . Functional balance between T cell chimeric receptor density and tumor associated antigen density: CTL mediated cytolysis and lymphokine production Gene Ther 2000 7: 35–42
Takahashi K, Ono K, Hirabayashi Y, Taniguchi M . Escape mechanisms of melanoma from immune system by soluble melanoma antigen J Immunol 1988 140: 3244–3248
Banat GA, Christ O, Cochlovirus B, Pralle HB, Zoller M . Tumour-induced suppression of immune response and its correction Cancer Immunol Immunother 2001 49: 573–586
Gregorian SK, Battisto JR . Immunosuppression in murine renal cell carcinoma: I. Characterization of extent, severity and sources Cancer Immunol Immunother 1990 31: 325–334
Gregorian SK, Battisto JR . Immunosuppression in murine renal cell carcinoma: II. Identification of responsible lymphoid cell phenotypes and examination of elimination of suppression Cancer Immunol Immunother 1990 31: 335–341
Cavallo F, Martin-Fontecha A, Bellone M et al. Co-expression of B7-1 and ICAM-1 on tumors is required for rejection and the establishment of a memory response Eur J Immunol 1995 25: 1154–1162
Kufer P et al. Construction and biological activity of a recombinant bispecific single-chain antibody designed for therapy of minimal residual colorectal cancer Cancer Immunol Immunother 1997 45: 193–197
Richards J, Auger J, Peace D et al. Phase I evaluation of humanized OKT3: toxicity and immunomodulatory effects of hOKT3gamma4 Cancer Res 1999 59: 2096–2101
Gilboa E . How tumors escape immune destruction and what we can do about it Cancer Immunol Immunother 1999 48: 382–385
Acknowledgements
The authors sincerely thank K Dott and J Kintz for technical assistance, and P Slos and P Erbs for help in statistical analysis. This work was supported, in part, by the Convention Industrielle pour la Formation par la Recherche CIFRE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, S., Regulier, E., Rooke, R. et al. Tumor gene therapy by MVA-mediated expression of T-cell–stimulating antibodies. Cancer Gene Ther 9, 470–477 (2002). https://doi.org/10.1038/sj.cgt.7700461
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700461
Keywords
This article is cited by
-
Oncolytic viruses as engineering platforms for combination immunotherapy
Nature Reviews Cancer (2018)
-
Adenovirus-mediated intratumoral expression of immunostimulatory proteins in combination with systemic Treg inactivation induces tumor-destructive immune responses in mouse models
Cancer Gene Therapy (2011)
-
Secretomers as a new tool for the monitoring of CTL responses
Cancer Immunology, Immunotherapy (2005)
-
Cancer gene therapy: an awkward adolescence
Cancer Gene Therapy (2003)
-
Stable expression of chimeric anti-CD3 receptors on mammalian cells for stimulation of antitumor immunity
Cancer Gene Therapy (2003)