Abstract
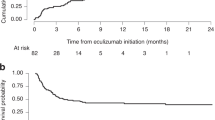
Veno-occlusive disease (VOD) is a common and high-risk complication of allogeneic stem cell transplantation (SCT). Defibrotide has recently been used successfully to treat the disorder. We report on 58 patients who received defibrotide prophylaxis without concurrent heparin. No patients fulfilled the Baltimore criteria for VOD or died of the condition within 100 days of SCT. None of this group developed haemorrhagic complications secondary to defibrotide. These observations suggest that prophylaxis with defibrotide alone may reduce the incidence of VOD post-SCT although a randomised controlled trial is warranted to further evaluate its role.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB . Veno-occlusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Onc 1993; 11: 1729–1736.
Deleve LD, Shulman HM, McDonald GB . Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22: 27–42.
Shulman HM, Fisher LB, Schoch HG, Henne KW, McDonald GB . Veno-occlusive disease of the liver after marrow transplantation: histological correlates of clinical signs and symptoms. Hepatology 1994; 19: 1171–1181.
Salat C, Holler E, Kolb HJ, Reinhardt B, Pihusch R, Wilmanns W et al. Plasminogen activator inhibitor-1 confirms the diagnosis of hepatic veno-occlusive disease in patients with hyperbilirubinaemia after bone marrow transplantation. Blood 1997; 89: 2184–2188.
Carreras E, Bertz H, Arcese W, Vernant JP, Tomas JF, Hagglund H et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukaemia Working Party. Blood 1998; 92: 3599–3604.
Ruutu T, Eriksson B, Remes K, Juvoven E, Volin L, Remberger M et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood 2002; 100: 1977–1983.
Richardson PG, Elias AD, Krishnan A, Wheeler C, Nath R, Hoppensteadt D et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood 1998; 92: 737–744.
Chopra R, Eaton JD, Grassi A, Potter M, Shaw B, Salat C et al. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol 2000; 111: 1122–1129.
Richardson PG, Murakami C, Jin Z, Warren E, Momtaz P, Hoppensteadt D et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high risk population and factors predictive of outcome. Blood 2002; 100: 4337–4343.
Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant 2004; 33: 189–195.
Richardson PG, Soiffer RJ, Antin JH, Jin Z, Kurtzberg J, Martin PL et al. Defibrotide for the treatment of veno-occlusive disease and multi-system organ failure post SCT: analysis of response and survival according to degree and type of MOF. Blood 2005; 106: 11 (abstract 399).
Haussmann U, Fischer J, Eber S, Scherer F, Seger R, Gungor T et al. Hepatic veno-occlusive disease in pediatric stem cell transplantation: impact of pre-emptive antithrombin III replacement and combined antithrombin III/defibrotide therapy. Haematologica 2006; 91: 795–800.
Imran H, Tleyjeh IM, Zirakzadeh A, Rodriguez V, Khan SP . Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transplant 2006; 37: 677–686.
Bearman S, Shen DD, Hinds MS, Hill HA, McDonald G . A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br J Haematol 1993; 84: 724–730.
Ohasi K, Tanabe J, Watanbe R, Tanaka T, Sakamahi H, Maruta A et al. The Japanese multicenter open randomised trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol 2000; 64: 32–38.
Chalandon Y, Roosnek E, Mermillod B, Newton A, Ozsabin H, Wacker P et al. Prevention of veno-occlusive disease with defibrotide after allogenic stem cell transplantation. Biol Blood Marrow Transplant 2004; 10: 347–354.
Corbacioglu S, Honig M, Lahr G, Stohr S, Berry G, Friedrich W et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant 2006; 38: 547–553.
Jones RJ, Lee KSK, Beschorner WE, Vogel VG, Grochow LB, Braine HB et al. Venocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N . Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse effects or liver function test abnormalities. J Clin Pharmacol 2006; 46: 235–243.
Rodriguez LAG, Duque A, Castellsague J, Perez-Gutthann S, Stricker BHCh . A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. J Clin Pharmacol 1999; 48: 847–852.
Andrade RJ, Lucena MI, Fernandez MC, Palaez G, Pachkena K, Garcia-Ruiz E et al. Drug induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10 year period. Gastroenterology 2005; 129: 512–521.
Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W et al. Second allogeneic marrow transplantation for patients with recurrent leukaemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol 1993; 11: 304–313.
Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica 2005; 90: 1396–1404.
De Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily busulphan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Van Besien K, Devine S, Wickrema A, Jessop E, Amin K, Yassine M et al. Regimen-toxicity after fludarabine-melphalan conditioning: a prospective study of 31 patients with hematologic malignancies. Bone Marrow Transplant 2003; 32: 471–476.
Acknowledgements
No external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dignan, F., Gujral, D., Ethell, M. et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from veno-occlusive disease. Bone Marrow Transplant 40, 79–82 (2007). https://doi.org/10.1038/sj.bmt.1705696
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705696
Keywords
This article is cited by
-
A Systematic Review and Meta-Analysis of Studies of Defibrotide Prophylaxis for Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome
Clinical Drug Investigation (2022)
-
Efficacy of low dose and short duration defibrotide prophylaxis for hepatic veno-occlusive disease after autologous haematopoietic stem cell transplantation
Bone Marrow Transplantation (2021)
-
Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group
Bone Marrow Transplantation (2020)
-
Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS)
Bone Marrow Transplantation (2019)
-
Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation
Bone Marrow Transplantation (2018)