Abstract
Background:
The aim of this study was to reveal the clinicopathological and molecular characteristics of microsatellite instability-high (MSI-H) colorectal cancers (CRCs) showing programmed death ligand-1 (PD-L1) positivity, which are good candidates for anti-PD-1/PD-L1 immunotherapy.
Methods:
The PD-L1 expression status of 208 MSI-H CRCs was retrospectively analysed using immunohistochemistry. PD-L1 positivity in tumour cells (PD-L1+(T)) and PD-L1 positivity in immune cells (PD-L1+(I)) were separately evaluated.
Results:
Programmed death ligand-1 positivity in tumour cells and PD-L1+(I) were observed in 26 (12.5%) and 62 (29.8%) MSI-H CRCs, respectively, and occasionally overlapped (n=12; 5.8%). Programmed death ligand-1 positivity tumours in MSI-H CRCs were significantly associated with older age, female sex, non-mucinous-type poor differentiation, infiltrating growth, tumour budding, advanced stage, CpG island methylator phenotype-high, MLH1 promoter methylation, and BRAF V600E mutations. However, PD-L1+(I) MSI-H CRCs were characterised by high-density tumour-infiltrating immune cells, including T cells and macrophages, and intense peritumoural lymphoid reactions. In patients with stage IV MSI-H CRCs who had undergone metastatectomy (n=4), the PD-L1 status of primary tumours was maintained in corresponding distant metastatic lesions.
Conclusions:
In MSI-H CRCs, PD-L1+(T) and PD-L1+(I) are linked to a sporadic hypermethylated subtype and an immune cell-rich subtype, respectively. Potential differential therapeutic implications of PD-L1+(T) and PD-L1+(I) in CRCs should be further investigated.
Similar content being viewed by others
Main
Immunotherapy using immune checkpoint inhibitors is a rapidly growing modality for the treatment of human cancers. The programmed death-1 (PD-1) receptor and its ligand, programmed death ligand-1 (PD-L1), have been tested as representative major immune checkpoint targets for immunotherapy in various malignancies (Homet Moreno and Ribas, 2015). In the tumour immune microenvironment, the PD-1/PD-L1 signalling axis may induce immune inhibitory/exhaustion signalling to activated T cells, and thus the antitumour immune response is significantly impaired. To reverse this unfavourable condition, several blockades of the PD-1/PD-L1 pathway have been developed and are being tested. Theoretically, PD-1/PD-L1 blockades can recover the native antitumour function of T cells and can facilitate tumour regression by derepressed antitumour immunity. In recent years, based on relatively superior efficacy and safety, immune checkpoint inhibitors have been taking centre stage as promising cancer therapeutics in clinical settings.
Immunotherapy with antibodies against PD-1 or PD-L1 has been revealed to be effective in different tumour types, especially malignant melanomas, non-small-cell lung cancers, renal cell carcinomas, and bladder cancers (Brahmer et al, 2012 ; Topalian et al, 2012; Homet Moreno and Ribas, 2015). However, in colorectal cancers (CRCs), it has been demonstrated that a substantial number of tumours are unresponsive to anti-PD-1/PD-L1 therapy (Brahmer et al, 2012; Topalian et al, 2012). Importantly, Llosa et al (2015) have discovered that a subset of CRCs, mainly microsatellite instability-high (MSI-H) CRCs, are closely associated with high expression of PD-1/PD-L1. Because previous investigations have suggested that high PD-L1 expression is associated with good responsiveness to anti-PD-1/PD-L1 therapy in cancers (Herbst et al, 2014; Taube et al, 2014), the MSI-H phenotype has been expected to be a potential candidate for anti-PD-1/PD-L1 therapy among CRCs (Xiao and Freeman, 2015). Moreover, a growing body of evidence suggests that not only PD-L1 expression but also tumour-infiltrating T cells may be important for responding well to anti-PD-1/PD-L1 therapy in cancers (Teng et al, 2015). Because MSI-H CRCs have been known to be associated with high-density tumour-infiltrating lymphocytes and frequent peritumoural lymphoid reactions, there is also a good chance that these tumours are good candidates for anti-PD-1/PD-L1 therapy. As expected, a recent phase 2 clinical trial has confirmed that PD-1 blockade can be beneficial in CRCs carrying DNA mismatch repair (MMR) deficiency, a causative molecular alteration of MSI-H (Le et al, 2015).
Although emerging evidence indicates that MSI-H CRCs will be good responders to anti-PD-1/PD-L1 immunotherapy (Lote et al, 2015; Dudley et al, 2016), high PD-L1 expression is found in a subset of MSI-H CRCs, but not in all MSI-H CRCs; therefore, responses to PD-1/PD-L1 blockades may be unequal among MSI-H CRCs. Therefore, it will be important to understand in detail which clinicopathological and molecular features characterise the subgroup of MSI-H CRCs showing PD-L1-positivity, indicating potential good responders to PD-1/PD-L1 blockades. In our study, we immunohistochemically evaluated PD-L1 expression status and statistically analysed its associations with various clinical, pathological and molecular parameters in a large series of MSI-H CRCs.
Materials and Methods
Collection of MSI-H CRCs
A total of 208 MSI-H CRC cases were included in this study, all of which had been collected from our previous studies (Kim et al, 2015b, 2015c). Formalin-fixed, paraffin-embedded (FFPE) tissues of the 208 MSI-H CRCs (208 primary tumour tissues and 4 distant metastatic tumour tissues) were retrieved from the pathology archives of the Seoul National University Hospital (Seoul, Korea) and the Seoul National University Bundang Hospital (Seongnam, Korea). All of the tumours had been surgically resected in our hospitals between 2004 and 2008 and were determined to be MSI-H tumours by our molecular pathology laboratory. DNA extraction from paired normal and tumour tissues of the 208 MSI-H CRCs and subsequent MSI analysis using fluorescent multiplex PCR with five NCI recommended markers (BAT-25, BAT-26, D5S346, D17S250, and D2S123) were performed as previously described (Kim et al, 2015b). All of the 208 MSI-H CRCs were also confirmed to be DNA MMR-deficient by immunostaining for MMR proteins (MLH1/MSH2/MSH6/PMS2). In our study, the 208 MSI-H CRCs were classified into sporadic or suspected hereditary cases based on molecular profiles. Sporadic MSI-H CRCs were defined as having at least one of the following three molecular features: CpG island methylator phenotype-high (CIMP-H), promoter methylation of the MLH1 gene, or BRAF V600E mutation. Cases that did not fulfil the above criteria were determined to be suspected Lynch syndrome (LS)-associated MSI-H CRCs. This study was approved by the institutional review board (SNUH IRB H-1203-072-402).
Clinical and histopathological data
Clinical data of the 208 MSI-H CRCs were retrospectively collected by medical record review (Kim et al, 2015b, 2015c). The data included age, gender, tumour location, tumour multiplicity, gross tumour type, American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM stage, and disease-free survival. Evaluation of the histopathological features of the 208 MSI-H CRCs was conducted as previously described (Kim et al, 2015b, 2015c). The histological parameters assessed were depth of invasion (pT category), lymph node metastasis (pN category), tumour differentiation (WHO tumour grade), invasive growth pattern, mucinous histology, signet ring cell histology, medullary histology, serrated histology, lymphovascular invasion, perineural invasion, tumour budding, peritumoural lymphoid reaction, and Crohn-like lymphoid reaction. Detailed criteria for some of the histological parameters, including tumour differentiation, mucinous histology, signet ring cell histology, medullary histology, serrated histology, tumour budding, peritumoural lymphoid reaction, and Crohn-like lymphoid reaction, were based on our previous investigation (Kim et al, 2015a). Invasive growth pattern was evaluated according to the Jass’s original criteria (Jass et al, 1996).
Immunohistochemistry
For high-throughput immunohistochemical analysis, tissue microarray (TMA) was constructed using FFPE tissues of 208 MSI-H CRCs as previously described (Kim et al, 2015b). Three TMA cores (2 mm in diameter) were extracted from three different areas of a representative tumour block of each case. Immunohistochemistry (IHC) was performed on TMA sections using antibodies against PD-L1 (E1L3N; 1:50; Cell Signaling Technology, Danvers, MA, USA), CD3 (Leica Biosystems, Novocastra, Newcastle-upon-Tyne, UK), CD68 (Leica Biosystems, Novocastra), MLH1 (Dako, Carpinteria, CA, USA), MSH2 (Invitrogen, Camarillo, CA, USA), MSH6 (Ventana Medical Systems, Tucson, AZ, USA), and PMS2 (Ventana Medical Systems). All IHC staining procedures were conducted using automated immunostainers (Ventana BenchMark XT for PD-L1, MLH1, MSH2, MSH6, and PMS2; Dako Omnis for CD3 and CD68) according to the manufacturers’ protocols. Microscopic interpretation of each IHC marker was performed with the following criteria: (1) PD-L1 was determined to be positive in tumour cells (PD-L1+(T)) when moderate (2+) or strong (3+) intensity of the membranous-to-cytoplasmic staining pattern of PD-L1 was shown in more than 5% of the tumour cells, and PD-L1 was determined to be positive in immune cells (PD-L1+(I)) when moderate (2+) or strong (3+) intensity of the membranous-to-cytoplasmic staining pattern of PD-L1 was shown in more than 5% of immune cells. Cell percentage criteria were based on the total area of the three TMA cores of each case. (2) Numbers of CD3-positive cells (CD3+ cells, indicating tumour-infiltrating T lymphocytes) and CD68-positive cells (CD68+ cells, indicating tumour-infiltrating macrophages) per high power field were manually counted under × 400 magnification in the most intense area of a TMA core. The average of the densities of CD3+ cells or CD68+ cells counted from the three TMA cores of each case was regarded as the final density of CD3+ cells or CD68+ cells of the case. (3) DNA MMR proteins (MLH1, MSH2, MSH6, and PMS2) were determined to be positive in a case when nuclear staining in the tumour cells of the case was observed. Loss of expression (negative) of MMR proteins was determined when nuclear staining in the tumour cells was not observed. Among the 208 MSI-H CRCs, 2 and 11 cases were excluded from the CD3 and CD68 analyses, respectively, owing to the suboptimal quality of the TMA core sections.
DNA analysis
Major molecular parameters of CRC, including the CpG island methylator phenotype (CIMP) and mutations of the KRAS (codons 12 and 13) and BRAF (codon 600) genes, were analysed using DNA samples isolated from the 208 primary MSI-H CRC tissues as previously described (Kim et al, 2015b). CpG island methylator phenotype was determined using eight CIMP markers (MLH1, NEUROG1, CRABP1, CACNA1G, CDKN2A, IGF2, SOCS1, and RUNX3), and the promoter CpG island methylation of each marker gene was quantitatively measured by real-time PCR (MethyLight assay). CIMP status in each tumour was classified into one of the three phenotypes, including CIMP-high (CIMP-H), CIMP-low (CIMP-L), and CIMP-negative (CIMP-0). KRAS/BRAF mutations were analysed by allele-specific PCR and Sanger sequencing. Among the 208 MSI-H CRCs, 7 cases were excluded from KRAS mutation analysis, owing to an insufficient amount or poor quality of the isolated DNA.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistics version 20 (IBM, Armonk, NY, USA). The χ2-test or Fisher’s exact test was used for the comparison of categorical variables. To compare the differences in disease-free survival between PD-L1-positive and PD-L1-negative subgroups, survival analysis was performed using the Kaplan–Meier method with the log-rank test. All P-values were two-sided, and P<0.05 was considered to be statistically significant.
Results
PD-L1 expression status in MSI-H CRCs
Immunohistochemical expression of PD-L1 was observed in tumour cells and/or immune cells in subsets of MSI-H CRCs. PD-L1-positivity in tumour cells (PD-L1+(T); 2+ or 3+ intensity in >5% of tumour cells) was detected in 26 of 208 MSI-H CRCs (12.5%), and PD-L1-positivity in immune cells (PD-L1+(I); 2+ or 3+ intensity in>5% of immune cells) was detected in 62 of 208 MSI-H CRCs (29.8%). MSI-H CRCs exhibiting both PD-L1+(T) and PD-L1+(I) rarely existed (12 cases; 5.8%).
Characteristics of a PD-L1+(T) subset of MSI-H CRCs
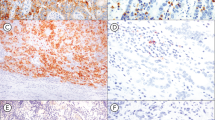
Compared with tumoural PD-L1-negative (PD-L1−(T)) MSI-H CRCs, PD-L1+(T) MSI-H CRCs were characterised by distinct clinicopathological and molecular features (Table 1). Clinicopathologically, PD-L1+(T) tumours in MSI-H CRCs significantly correlated with older age of onset (73%), female sex (73%), infiltrating growth pattern (58%), advanced AJCC/UICC stage (stage III/IV; 54%), lymphovascular invasion (46%), high-density tumour-infiltrating T cells (81%), high-density tumour-infiltrating macrophages (71%), poor differentiation (65%), tumour budding-positivity (46%), and a lack of mucinous histology (65%). However, other histological features such as signet ring cell histology and medullary histology were not associated with tumoural PD-L1 expression status (Supplementary Table S1). In addition, PD-L1+(T) tumours in MSI-H CRCs were molecularly associated with loss of expression of MLH1/PMS2 (both 92%), CIMP-H status (58%), MLH1 methylation (65%), and BRAF V600E mutation (23%). Collectively, PD-L1+(T) was significantly correlated with molecularly defined sporadic MSI-H CRCs (69%). In the Kaplan–Meier survival analysis, the PD-L1+(T) subgroup showed a tendency towards worse prognosis than the PD-L1−(T) subgroup of MSI-H CRCs, but statistical significance was not observed (log-rank P=0.114; Supplementary Figure S1A). Representative images of PD-L1+(T) and matched non-mucinous-type poorly differentiated histology in an MSI-H CRC are presented in Figure 1A and B.
Immunohistochemical expression of PD-L1 and its associated histological features in MSI-H CRC. (A and B) Representative photomicrographs of PD-L1+(T) (A; IHC, × 200) and its matched non-mucinous-type poorly differentiated histology (B; H&E, × 200) in an MSI-H CRC. (C and D) Representative photomicrographs of PD-L1+(I) (C; IHC, × 200) and its matched peritumoural lymphoid reaction (D; H&E, × 100) in an MSI-H CRC. (E and F) Representative photomicrographs of PD-L1+(T)/PD-L1+(I) in a primary tumour (E; IHC, × 200) and a corresponding metastatic lesion (F; IHC, × 200) in a stage IV MSI-H CRC.
Characteristics of a PD-L1+(I) subset of MSI-H CRCs
Differential features between PD-L1+(I) and PD-L1−(I) MSI-H CRCs are summarised in Table 2. Programmed death ligand-1 positivity in immune cells MSI-H CRCs were significantly correlated with early stage (stage I/II; 81%), moderate to marked peritumoural lymphoid reaction (77%), high-density tumour-infiltrating T cells (77%), high-density tumour-infiltrating macrophages (70%), and a lack of mucinous histology (55%). However, PD-L1+(I) was not associated with CIMP status or hereditary/sporadic type (Supplementary Table S2). In the Kaplan–Meier survival analysis, the PD-L1+(I) subgroup showed a tendency towards better prognosis than the PD-L1−(I) subgroup of MSI-H CRCs, but statistical significance was not reached (log-rank P=0.142; Supplementary Figure S1B). Representative images of PD-L1+(I) and matched intense peritumoural lymphoid reaction in an MSI-H CRC are presented in Figure 1C and D.
Comparison of PD-L1 expression between primary and metastatic MSI-H CRCs
Among stage IV MSI-H CRCs (n=18), four cases had received surgical resection of distant metastatic lesions. PD-L1 expression status was compared between primary tumour tissues and corresponding distant metastatic tumour tissues of these four stage IV MSI-H CRCs. In one of these four cases, the primary tumour showed PD-L1+(T)/PD-L1+(I), but the other three cases were completely negative for PD-L1 expression (PD-L1−(T)/PD-L1−(I)) in their primary tumours. As expected, positive expression of PD-L1 in tumour cells and immune cells was observed in the corresponding distant metastatic lesion of the primary PD-L1+(T)/PD-L1+(I) MSI-H CRC (Figures 1E and F), whereas PD-L1-negativity was maintained in the corresponding distant metastatic lesions of the primary PD-L1−(T)/PD-L1−(I) MSI-H CRCs.
Discussion
Recent evidence has revealed that MSI-H CRCs can be potential good responders to anti-PD-1/PD-L1 immunotherapy (Le et al, 2015; Llosa et al, 2015; Lote et al, 2015; Dudley et al, 2016). However, to date, there is a lack of data regarding the detailed profile of PD-L1 expression and its clinicopathological and molecular implications in CRCs. To the best of our knowledge, our present study is the first report to identify underlying differential characteristics of PD-L1 expression between tumour cells and immune cells in MSI-H CRCs. Our study provides in-depth data concerning clinical, pathological, and molecular associations of PD-L1+(T) and PD-L1+(I) in MSI-H CRCs, which may be helpful for guiding future directions of basic studies and clinical applications of anti-PD-1/PD-L1 immunotherapy in CRC. Several key points from the results of this study are as follows:
In terms of histopathological features, PD-L1+(T) was significantly associated with poor differentiation (tumour grade 3), decreased extracellular mucin component, infiltrating growth pattern, tumour budding positivity, increased lymphovascular invasion, and advanced stage in MSI-H CRCs (Table 1). These findings indicate that PD-L1 expression in tumour cells is closely linked to aggressive histological features in CRC. Interestingly, a recent communication by Prall et al. has suggested that tumour budding is associated with PD-L1 expression in CRCs (Prall and Huhns, 2015). In addition, a recent article by Zhu et al (2015) has also reported that high PD-L1 expression in tumour cells is significantly associated with nodal and distant metastases and poor prognosis in serrated CRCs. In our study, the PD-L1+(T) group demonstrated a tendency towards worse disease-free survival than the PD-L1−(T) group of MSI-H CRCs (Supplementary Figure S1A). The poor prognostic impact of PD-L1+(T) in MSI-H CRCs may be a reflection of aggressive pathological features of PD-L1+(T) tumours.
Several mechanisms of PD-L1 upregulation in tumour cells have been suggested. Representative regulatory factors of PD-L1 expression in tumour cells include the MAPK signalling pathway, the PI3K/Akt signalling pathway, and transcription factors such as HIF-1, STAT3, and NF-κB (Chen et al, 2016). In general, PD-L1 can be expressed on activated inflammatory cells and on epithelial cells and is upregulated by the proinflammatory cytokine interferon (IFN)-γ, which is produced by activated T cells (Topalian et al, 2015). The intense infiltration of active cytotoxic T cells and activated Th1 cells in the tumour immune microenvironment of MSI-H CRCs (Llosa et al, 2015; Xiao and Freeman, 2015) could explain the frequent PD-L1 upregulation in these MSI-H CRCs, which may occur due to high IFN-γ production by such abundant tumour-infiltrating T cells. More fundamentally, MSI-H CRCs generally harbour higher tumour-specific neoantigen loads than do microsatellite-stable/MSI-low CRCs, because MSI-H status essentially induces frequent genome-wide microsatellite mutations in tumours. High neoantigen loads in MSI-H CRCs can induce active antitumour immune responses including intense infiltration of active cytotoxic T cells and activated Th1 cells. According to a previous study by Howitt et al (2015), both PD-L1 expression and neoantigen load are significantly increased in MSI-H endometrial cancers. Therefore, it might have been expected that most MSI-H CRCs might express high PD-L1; however, we found that only subsets of MSI-H CRCs were PD-L1+(T) and/or PD-L1+(I). Thus, a high neoantigen load or a high infiltration of activated T cells cannot solely explain the PD-L1 upregulation observed in MSI-H CRC subsets. Importantly, in our study results, a substantial number of PD-L1+(T) tumours were CIMP-H/MSI-H CRCs (Table 1). Although a significant difference in neoantigen loads between CIMP-H/MSI-H CRCs and CIMP-L/0/MSI-H CRCs has not been proven, production of critical neoantigen loads for PD-L1 upregulation may be facilitated by yet-unknown epigenetic mechanisms in CIMP-H/MSI-H CRCs rather than in CIMP-L/0 MSI-H CRCs. Potential mechanisms and clinicopathological and therapeutic implications of PD-L1 expression in MSI-H CRCs are further discussed below.
The underlying mechanism of PD-L1 upregulation in CIMP-H/MSI-H CRC cells with aggressive histology, especially in those with poor differentiation, infiltrating growth, and tumour budding, is unclear. However, several previous studies investigating PD-L1 in other malignancies such as non-small-cell lung cancer, renal cell carcinoma, and breast cancer have provided important clues that increased epithelial–mesenchymal transition (EMT) features may be associated with PD-L1 expression in tumour cells (Chen et al, 2014; Alsuliman et al, 2015; Wang et al, 2015). Because poor differentiation, infiltrating growth, and tumour budding are regarded to be partially based on EMT, the observed significant correlation between upregulated PD-L1 expression and poor differentiation/infiltrating growth/tumour budding in CRC may be a plausible finding.
Another potential mechanism of high PD-L1 expression in CIMP-H/MSI-H CRCs is activation of the MAPK signalling pathway. According to accumulating evidence, MAPK pathway activation by the BRAF V600E mutation drives PD-L1 expression in melanoma (Chen et al, 2016). Because it is well known that CIMP-H is tightly associated with BRAF mutation in CRCs, it can be hypothesised that the observed association between PD-L1+(T) and CIMP-H in MSI-H CRCs may be based on the relationship between PD-L1 expression and BRAF mutation. However, in our results, although there was a significant association between PD-L1+(T) and BRAF mutation, BRAF mutation was found in only 23% of PD-L1+(T) MSI-H CRCs (Table 1). This finding indicates that PD-L1+(T) in MSI-H CRCs cannot be regulated solely by BRAF mutation. Genetic or epigenetic regulatory mechanisms of tumoural PD-L1 expression in CRCs should be further investigated.
The observed significant associations of PD-L1+(T) with older age, female gender, poor differentiation, CIMP-H, MLH1 methylation, and BRAF mutation collectively indicate that PD-L1 expression in tumour cells is specifically enriched in a sporadic hypermethylated subtype among MSI-H CRCs. To the best of our knowledge, any in vitro or in vivo evidence showing an association between tumoural PD-L1 expression and DNA methylation in CRCs has not been reported. Our novel finding has important therapeutic implications. Emerging evidence has revealed that epigenetic therapy, particularly DNA demethylating agent therapy, has a potential to be synergistically combined with immunotherapy, especially immune checkpoint blockade therapy, to efficiently treat cancers (Chiappinelli et al, 2016). This concept is based on experimental findings that DNA methyltransferase inhibitors can demethylate endogenous retroviruses and cancer testis antigens and may induce T-cell attraction, ultimately increasing immune checkpoint inhibitor efficacy (Chiappinelli et al, 2016). Therefore, sporadic MSI-H CRCs may be good candidates for this combination therapy, as these tumours are associated with both DNA hypermethylation and high PD-L1 expression. Future clinical studies of combined epigenetic and immunotherapy as a novel treatment strategy in sporadic MSI-H CRCs are warranted.
In terms of tumour immune microenvironment, we found that PD-L1+(I) MSI-H CRCs were significantly associated with high infiltration of T cells and macrophages. This finding corresponded to the previous results by Llosa et al (2015), in which MSI-H CRCs were characterised by both high PD-L1 expression in immune cells, especially in myeloid cells, and dense tumour-infiltrating lymphocytes. Because it has been suggested that a high density of tumour-infiltrating lymphocytes and peritumoural lymphoid reactions are closely associated with favourable prognoses in CRCs (Mei et al, 2014), the observation that PD-L1+(I) tumours were associated with early stage (Table 2) and showed a tendency towards better survival than PD-L1−(I) tumours in MSI-H CRCs seems reasonable (Supplementary Figure S1B). Xiao and Freeman (2015) have previously suggested hypotheses regarding potential causes of upregulation of PD-L1 expression in tumour-infiltrating immune cells of MSI-H CRCs, including high neoantigen load and the abundance of intratumoural gut microbiota such as Fusobacterium nucleatum in MSI-H CRCs. Future studies should focus on the identification of differential regulatory mechanisms and therapeutic responses of PD-L1+(T) and PD-L1+(I) in MSI-H CRCs.
Finally, according to our study, PD-L1 expression status was concordant between primary tumours and corresponding distant metastatic tumours in stage IV MSI-H CRCs (Figure 1E and F). However, the number of tested cases was very small (n=4), and, moreover, only one PD-L1+ case was included. Therefore, the ability of our results to be generalised is limited, and more extended studies comparing PD-L1 expression status between primary and metastatic lesions of CRCs are needed. For reference, although there is also little evidence in other malignancies, discordance of PD-L1 expression between primary and metastatic tumours has been observed in subsets of renal cell carcinomas, bladder cancers, and breast cancers (Callea et al, 2015; Cimino-Mathews et al, 2016; Mukherji et al, 2016).
In conclusion, PD-L1+(T) MSI-H CRCs are mainly composed of sporadic poorly differentiated CIMP-H tumours, whereas PD-L1+(I) MSI-H CRCs are characterised by dense intratumoural and peritumoural infiltration of immune cells. Although our study provides novel insights into the pathological and molecular bases of PD-L1 expression in CRCs, important questions remain concerning which genetic or epigenetic alterations could critically affect PD-L1 expression in CRCs and whether there would be a difference in response to anti-PD-1/PD-L1 therapy between PD-L1+(T) and PD-L1+(I) subsets of MSI-H CRCs.
Change history
09 August 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, Al-Alwan M, Ghebeh H (2015) Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer 14: 149.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366 (26): 2455–2465.
Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, Hodi FS, Choueiri TK, McDermott DF, Freeman GJ, Signoretti S (2015) Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res 3 (10): 1158–1164.
Chen J, Jiang CC, Jin L, Zhang XD (2016) Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 27 (3): 409–416.
Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal JD, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Jones S, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX (2014) Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 5: 5241.
Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB (2016) Combining epigenetic and immunotherapy to combat cancer. Cancer Res 76 (7): 1683–1689.
Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, Cuka N, Argani P, Emens LA (2016) PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 47 (1): 52–63.
Dudley JC, Lin MT, Le DT, Eshleman JR (2016) Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 22 (4): 813–820.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515 (7528): 563–567.
Homet Moreno B, Ribas A (2015) Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer 112 (9): 1421–1427.
Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC, D'Andrea AD, Wu CJ, Matulonis UA, Konstantinopoulos PA (2015) Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1 (9): 1319–1323.
Jass JR, Ajioka Y, Allen JP, Chan YF, Cohen RJ, Nixon JM, Radojkovic M, Restall AP, Stables SR, Zwi LJ (1996) Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology 28 (6): 543–548.
Kim JH, Bae JM, Oh HJ, Lee HS, Kang GH (2015a) Pathologic factors associated with prognosis after adjuvant chemotherapy in stage II/III microsatellite-unstable colorectal cancers. J Pathol Transl Med 49 (2): 118–128.
Kim JH, Bae JM, Song YS, Cho NY, Lee HS, Kang GH (2015b) Clinicopathologic, molecular, and prognostic implications of the loss of EPCAM expression in colorectal carcinoma. Oncotarget 7: 13372–13387.
Kim JH, Kim KJ, Bae JM, Rhee YY, Cho NY, Lee HS, Kang GH (2015c) Comparative validation of assessment criteria for Crohn-like lymphoid reaction in colorectal carcinoma. J Clin Pathol 68 (1): 22–28.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372 (26): 2509–2520.
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5 (1): 43–51.
Lote H, Cafferkey C, Chau I (2015) PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treat Rev 41 (10): 893–903.
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C (2014) Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 110 (6): 1595–1605.
Mukherji D, Jabbour MN, Saroufim M, Temraz S, Nasr R, Charafeddine M, Assi R, Shamseddine A, Tawil AN (2016) Programmed death-ligand 1 expression in muscle-invasive bladder cancer cystectomy specimens and lymph node metastasis: a reliable treatment selection biomarker? Clin Genitourin Cancer 14 (2): 183–187.
Prall F, Huhns M (2015) PD-L1 expression in tumour buds of colorectal carcinoma. Histopathology 69: 158–160.
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20 (19): 5064–5074.
Teng MW, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 75 (11): 2139–2145.
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27 (4): 450–461.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366 (26): 2443–2454.
Wang Y, Wang H, Zhao Q, Xia Y, Hu X, Guo J (2015) PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol 32 (8): 212.
Xiao Y, Freeman GJ (2015) The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov 5 (1): 16–18.
Zhu H, Qin H, Huang Z, Li S, Zhu X, He J, Yang J, Yu X, Yi X (2015) Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int J Clin Exp Pathol 8 (8): 9351–9359.
Acknowledgements
This study was supported by a grant from Basic Science Research Program through the National Research Foundation (NRF) funded by the Korean Ministry of Education (2013R1A1A2059080), a grant from the Korea Health Technology R&D Project funded by the Korean Ministry of Health and Welfare (HI13C1804), the NRF grant funded by the Korean Ministry of Science, ICT and Future planning (2011-0030049), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Korean Ministry of Health and Welfare (HI14C1277).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kim, J., Park, H., Cho, NY. et al. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer 115, 490–496 (2016). https://doi.org/10.1038/bjc.2016.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.211
Keywords
This article is cited by
-
Immunology of Lynch Syndrome
Current Oncology Reports (2021)
-
Programmed death ligand 1 (PD-L1) in colon cancer and its interaction with budding and tumor-infiltrating lymphocytes (TILs) as tumor-host antagonists
International Journal of Colorectal Disease (2021)
-
A novel NGS-based microsatellite instability (MSI) status classifier with 9 loci for colorectal cancer patients
Journal of Translational Medicine (2020)
-
Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway
Cell Death & Differentiation (2020)
-
Prevalence of PD-L1 expression is associated with EMAST, density of peritumoral T-cells and recurrence-free survival in operable non-metastatic colorectal cancer
Cancer Immunology, Immunotherapy (2020)