Abstract
Background:
Tumour budding is an adverse prognostic indicator in colorectal cancer (CRC). Marked overall peritumoural inflammation has been associated with favourable outcome and may lead to the presence of isolated cancer cells due to destruction of invading cancer cell islets.
Methods:
We assessed the prognostic significance of tumour budding and peritumoural inflammation in a cohort of 381 patients with CRC applying univariate and multivariate analyses.
Results:
Patients with high-grade budding and marked inflammation had a significantly better outcome compared with patients with high-grade budding and only mild inflammation. Outcome in these cases, however, was still worse compared with cases with low-grade budding, in which the extent of peritumoural inflammation had no further prognostic effect.
Conclusions:
Tumour budding proved to be a powerful prognostic variable in patients with CRC. Scattering of invading cancer cell islets by marked overall peritumoural inflammation seems to have a minor role.
Similar content being viewed by others
Main
In colorectal cancer (CRC), the morphology at the invasive tumour margin is believed to reflect tumour dynamics with pro- and anti-tumour effects, thereby rendering important prognostic information (Zlobec and Lugli, 2010).
Tumour budding, defined as the presence of isolated single cells or small clusters of cells (composed of fewer than five cells) (Ueno et al, 2002; Prall, 2007), has repeatedly been associated with adverse outcome (Ueno et al, 2002; Shinto et al, 2006; Choi et al, 2007; Prall, 2007; Kanazawa et al, 2008; Ohtsuki et al, 2008; Betge et al, 2012; Lugli et al, 2012; Karamitopoulou et al, 2013), while marked peritumoural (and intratumoural) inflammation has been associated with favourable outcome (Klintrup et al, 2005). Of note, the anti-tumour activity of inflammation, particularly lymphocytes and neutrophils, may lead to the destruction of tumour glands and, ultimately, the presence of isolated cells or small clusters of cells scattered in the tumour stroma, which has to be differentiated from true tumour budding.
Our study aimed to analyse the relationship between tumour budding and peritumoural inflammation in a large cohort of CRC patients. Specifically, we were interested to answer the question whether patients with both high-grade budding and high-grade inflammation have a prognosis comparable to patients with low-grade budding.
Materials and methods
Study cohort
The study cohort comprised 381 patients (166 males, 215 females; median age 70.1 years) and has been described in detail elsewhere (Harbaum et al, 2015).
Decision for adjuvant therapy was based upon American Joint Committee on Cancer (AJCC)/Union Internationale Contre le Cancer (UICC) tumour stage. Thus, patients with stage III disease received 5-fluorouracil/folinic acid following the Mayo Clinic recommendations (Moertel et al, 1990), while patients lacking nodal metastasis (stages I and II) remained without adjuvant treatment. Patients with node-positive rectal cancer received adjuvant radiotherapy. Patients who underwent neoadjuvant therapy were excluded.
The follow-up regimen, which included laboratory testing, abdominal ultrasound, chest X-ray as well as pelvic computerized tomography for rectum cancer patients, has been referred to in detail in our previous publication (Harbaum et al, 2015). Local or systemic relapse defined disease progression. Patients were followed after resection until death or time of last follow-up. The Ethics Committee of the Medial University of Graz, Austria, provided institutional Review Board approval.
Histopathology
All histological slides were independently reviewed by two gastrointestinal pathologists (MJP and CL), who were unaware of clinical, in particular follow-up data. Tumour stage was assessed based upon the 7th edition of the AJCC/UICC TNM classification (Sobin et al, 2010).
The extent of tumour budding was assessed on haematoxylin- and eosin-stained slides as described previously (Betge et al, 2012). In the tumour area with highest budding intensity, the number of isolated single cells or small clusters of less than five cells at the invasive tumour margin was counted at × 200 magnification (field of vision: 0.95 mm2; Olympus BX45, × 20/0.40, Tokyo, Japan). For statistical comparison, tumours were divided into two groups based upon the number of budding foci: counts of 0–9 were classified as low-grade, while counts of 10 or more foci were classified as high-grade budding (Ueno et al, 2002; Betge et al, 2012).
The overall inflammatory reaction and the amount of lymphoid cells, neutrophilic and eosinophilic granulocytes as well as macrophages were assessed using the four-degree scale introduced by Klintrup et al (2005): a score 0 indicated ‘no increase of inflammatory cells’. Score 1 indicated ‘mild and patchy increase of inflammatory cells at the invasive margin, but no destruction of invading cancer cell islets by the inflammatory cells’. A score of 2 was given when inflammatory cells formed a ‘band-like infiltrate at the invasive margin with some destruction of cancer cell islets by inflammatory cells’. A score of 3 indicated a ‘very prominent inflammatory reaction, forming a cup-like zone at the invasive margin, and destruction of cancer cell islets was frequent and invariably present’.
Statistical analysis
Associations between tumour budding and other tumour features were analysed using the χ2-test. Progression-free and cancer-specific survival was determined using the Kaplan–Meier method and compared by the log-rank test. For multivariable testing, Cox’s proportional hazards regression models were performed. All P-values were two sided with significance at P<0.05. All statistical procedures were done with SPSS version 20.0 software (IBM, Armonk, NY, USA). This study complied with the REporting recommendations for tumour MARKer prognostic studies (REMARK) criteria (McShane et al, 2005).
Results
Overall, 221 (58%) tumours showed low-grade, 160 (42%) tumours high-grade budding. The extent of tumour budding was associated positively with T and N classification and tumour grade, however, inversely with the extent of peritumoural inflammation (Table 1). In all, 167 (44%) tumours showed no increase or only a mild and patchy increase of inflammatory cells at the invasive tumour margin, but no destruction of invading cancer cell islets (scores 0–1), whereas in 214 (56%) tumours a band-like mixed inflammatory infiltrate with at least some destruction of cancer cell islets was seen (scores 2–3). When analysis was restricted to tumours with high-grade budding, 82 (51%) cases showed mild inflammation (scores 0–1) and 78 (49%) marked inflammation (scores 2–3; Figure 1).
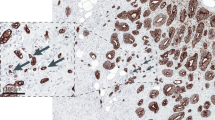
Tumour budding, defined as the presence of isolated single cells or small clusters of cells (composed of fewer than five cells), in a case with no increase of inflammatory cells at the invasive tumour margin, illustrated in serial sections stained with haematoxylin and eosin ( A ; original × 100) and anti-cytokeratin immunohistochemistry ( B ; original × 100). Marked peritumoural inflammation, leading to the presence of isolated cancer cells due to destruction of invading cancer cell islets, is shown in serial sections stained with haematoxylin and eosin (C; original × 100) and anti-cytokeratin immunohistochemistry (D; original × 100).
Follow-up information was available for 350 (92%) patients (median 45 months, range 1–182). In all, 141 (37%) patients developed progressive disease, and 118 (34%) died from cancer.
Tumour budding was significantly associated with poor progression-free (P<0.001) and cancer-specific (P<0.001) survival in our cohort. In multivariate analyses, tumour budding proved to be a predictor of both progression-free (hazard ratio (HR) 1.67, 95% confidence interval (CI) 1.11–2.51; P=0.014) and cancer-specific (HR 1.93, 95% CI 1.25–2.97; P=0.003) survival, independent of T and N classification, tumour grade, lymphatic and venous invasion. When peritumoural inflammation was added to analysis, tumour budding retained statistical significance, whereas for inflammation no independent impact on outcome was noted (data not shown).
Combined analysis of tumour budding and inflammatory cell reaction lead to the following results: patients with high-grade budding and marked inflammation had a better outcome, with respect to both progression-free (P<0.001) and cancer-specific survival (P<0.001), than patients with high-grade budding and only mild inflammation. Outcome in these cases, however, was still worse compared with patients with low-grade budding, in which the extent of peritumoural inflammation had no further prognostic effect (Figure 2). This was confirmed in multivariate analysis: in cancers with high-grade budding marked inflammation proved to be a predictor of favourable progression-free (HR 0.59, 95% CI 0.38–0.92; P=0.021) and cancer-specific (HR 0.58, 95% CI 0.36–0.93; P=0.024) survival, independent of T and N classification, tumour grade, lymphatic and venous invasion. In cancers with low-grade budding no independent impact on outcome was noted (data not shown).
Combined analysis of tumour budding and overall peritumoural inflammation. Patients with high-grade budding and marked inflammation had a better outcome, with respect to both progression-free (P<0.001) and cancer-specific survival (P<0.001), than patients with high-grade budding and only mild inflammation. Outcome in these cases, however, was still worse compared with patients with low-grade budding, in which the extent of peritumoural inflammation had no further prognostic effect.
Discussion
Tumour budding (single tumour cells or small tumour cell clusters) at the invasion front of CRC is an adverse prognostic indicator that has been linked to epithelial–mesenchymal transition, an important mechanism for cancer progression (Lugli et al, 2012). Ki67, caspase-3 and M30 staining is absent in most tumour buds, suggesting decreased proliferation and apoptosis (Dawson et al, 2014). Active immunosurveillance in the tumour microenvironment has been associated with low-frequency tumour budding and improved outcome. According to Koelzer et al (2015a), tumour buds may however evade immune recognition through downregulation of membranous major histocompatibility complex (MHC) class I, thereby conferring an aggressive phenotype with the potential to disseminate and metastasize (Zlobec and Lugli, 2010). On the molecular level, tumour budding is correlated inversely with microsatellite instability (MSI-H) (Zlobec et al, 2012).
Different methods to assess tumour budding have been introduced (Horcic et al, 2013; Karamitopoulou et al, 2013). Peritumoral inflammatory cells, including histiocytes, can be difficult to differentiate from tumour buds, and may sometimes obscure the underlying budding. Immunohistochemistry for anti-cytokeratin may help to highlight tumour buds in this setting (Mitrovic et al, 2012) and may also improve interobserver agreement (Koelzer et al, 2015b).
Marked peritumoural inflammation is a marker of favourable outcome in CRC (Klintrup et al, 2005; Roxburgh et al, 2009). As indicated above, the anti-tumour activity of the overall inflammatory cell reaction, driven mainly by neutrophils and macrophages, may lead to unspecific destruction of cancer cell islets, thereby generating isolated single cells or small clusters of cells. This phenomenon has to be differentiated, pathogenetically, from the specific anti-tumour response characterized by tumour-infiltrating T lymphocytes, which specifically target (pre-existing) tumour buds, described by Lugli et al (2009) as ‘nipping in the bud’.
In our study we were able to show that patients with high-grade budding and marked inflammation had a significantly better outcome compared with patients with high-grade budding and only mild inflammation, which may be due to inflammation-related destruction of cancer cell islets at the tumour margin. Outcome of cases with high-grade budding and marked inflammation, however, was still significantly worse compared with patients with low-grade budding. These data, which were evident in univariate and multivariate analyses, clearly confirm the outstanding value of tumour budding as a prognostic tool, independent of the inflammatory response.
Our study has strengths and limitations. The investigated cohort of CRC patients is large, with a comparably long follow-up time. Review pathology was performed independently by two experienced gastrointestinal pathologists. Foremost are the limitations inherent to retrospective analyses, and patients underwent surgery by multiple surgeons from both academic and community settings. In addition, budding was assessed only on haematoxylin and eosin stained slides, and anti-cytokeratin immunohistochemistry was not applied in this study.
In conclusion, high-grade budding assessed on haematoxylin and eosin stained slides is a powerful predictor of outcome in CRC. Inflammation-related destruction of invading cancer islets is able to stratify cases with high-grade budding into two distinct prognostic groups, but it seems to have a minor role in cases with low-grade budding.
Change history
16 February 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Betge J, Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Vieth M, Langner C (2012) Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann Surg Oncol 19: 3706–3712.
Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS (2007) Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis 22: 863–868.
Dawson H, Koelzer VH, Karamitopoulou E, Economou M, Hammer C, Muller DE, Lugli A, Zlobec I (2014) The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology 64: 577–584.
Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C (2015) Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol 28: 403–413.
Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, Lugli A (2013) Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol 44: 697–705.
Kanazawa H, Mitomi H, Nishiyama Y, Kishimoto I, Fukui N, Nakamura T, Watanabe M (2008) Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis 10: 41–47.
Karamitopoulou E, Zlobec I, Kölzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, Lugli A (2013) Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 26: 295–301.
Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ (2005) Inflammation and prognosis in colorectal cancer. Eur J Cancer 41: 2645–2654.
Koelzer VH, Dawson H, Andersson E, Karamitopoulou E, Masucci GV, Lugli A, Zlobec I (2015a) Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl Res 166: 207–217.
Koelzer VH, Zlobec I, Berger MD, Cathomas G, Dawson H, Dirschmid K, Hädrich M, Inderbitzin D, Offner F, Puppa G, Seelentag W, Schnüriger B, Tornillo L, Lugli A (2015b) Tumor budding in colorectal cancer revisited: results of a multicenter interobserver study. Virchows Arch 466: 485–493.
Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M, Terracciano L, Zlobec I (2009) CD8+ lymphocytes/tumour-budding index: an independent prognostic factor representing a 'pro-/anti-tumour' approach to tumour host interaction in colorectal cancer. Br J Cancer 101: 1382–1392.
Lugli A, Karamitopoulou E, Zlobec I (2012) Tumour budding: a promising parameter in colorectal cancer. Br J Cancer 106: 1713–1717.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93: 387–391.
Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R (2012) Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 25: 1315–1325.
Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322: 352–358.
Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, Nakagawa T, Uchimoto K, Nakamura S, Nonomura A, Nakajima Y (2008) Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res 28: 1831–1836.
Prall F (2007) Tumour budding in colorectal carcinoma. Histopathology 50: 151–162.
Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC (2009) Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur J Cancer 45: 2138–2145.
Shinto E, Jass JR, Tsuda H, Sato T, Ueno H, Hase K, Mochizuki H, Matsubara O (2006) Differential prognostic significance of morphologic invasive markers in colorectal cancer: tumor budding and cytoplasmic podia. Dis Colon Rectum 49: 1422–1430.
Sobin L, Gospodarowicz MK, Wittekind C (eds) (2010) TNM Classification of Malignant Tumors 7th edn. Wiley-Blackwell: West Sussex, UK.
Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC (2002) Tumour ‘budding' as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40: 127–132.
Zlobec I, Lugli A (2010) Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 1: 651–661.
Zlobec I, Bihl MP, Foerster A, Rufle A, Lugli A (2012) The impact of CpG island methylator phenotype and microsatellite instability on tumour budding in colorectal cancer. Histopathology 61: 777–787.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Max, N., Harbaum, L., Pollheimer, M. et al. Tumour budding with and without admixed inflammation: two different sides of the same coin?. Br J Cancer 114, 368–371 (2016). https://doi.org/10.1038/bjc.2015.454
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.454
Keywords
This article is cited by
-
Tumor infiltrative growth pattern correlates with the immune microenvironment and is an independent factor for lymph node metastasis and prognosis in stage T1 esophageal squamous cell carcinoma
Virchows Archiv (2020)
-
Automatic evaluation of tumor budding in immunohistochemically stained colorectal carcinomas and correlation to clinical outcome
Diagnostic Pathology (2018)
-
Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016
Modern Pathology (2017)