Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. Conventional cytotoxic chemotherapy has failed to show a substantial benefit for patients with HCC. Recently, a number of new drugs targeting molecular mechanisms involved in liver cell transformation have entered into clinical trials and led to encouraging results. In this review we summarise this data and point to a number of new compounds, which are currently being tested and can potentially broaden our therapeutic arsenal even further.
Similar content being viewed by others
Main
Hepatocellular carcinoma is the third most common cause of cancer-related death worldwide with 600 000 patients dying of this disease every year (Parkin et al, 2005). Although patients with early-stage disease have an excellent prognosis with a 5-year survival of more than 75–80%, there has been no effective therapy available for those with advanced disease, who are not eligible for transarterial chemoembolisation. We have reported earlier that 50% of HCC patients are diagnosed at an advanced stage (Greten et al, 2005). Recent advances in the development of targeted therapies have now also shown promising results. Here, we will discuss the results obtained using different molecular targeting agents and suggest new pathways for development of therapeutic options for HCC.
Molecular carcinogenesis
More than 80% of all HCC occur in patients with liver cirrhosis. Chronic viral infections as well as alcoholic liver disease are the most common etiologies of liver cirrhosis and also represent the initiation point of hepatocarcinogenesis (El-Serag and Rudolph, 2007). Continuous cell death, which induces the release of different cytokines, as well as fibrogenesis and development of liver cirrhosis have been identified as early factors responsible for carcinogenesis. Integration of hepatitis B virus (HBV) DNA into the host genome not only induces chromosomal instability, but depending on the site of DNA integration may also activate oncogenes or inactivate tumour-suppressor genes. Moreover, viral proteins (HBx and preS) have oncogenic properties. HBx activates different promoter elements and triggers activation of transcription factors such as AP-1 and NF-κB. Through this activity, genes, which are involved in cell cycle control, as well as apoptosis, are affected. A different molecular pathway has been suggested for hepatitis C virus (HCV)-induced hepatocarcinogenesis. In this case, HCV core protein affects cellular proliferation and apoptosis. In addition, chronic HCV infection can cause major immune responses, which might ultimately support hepatocarcinogenesis.
Tumour angiogenesis
Hepatocellular carcinoma is a highly vascularised tumour, which makes vascular targeting approaches appealing for the treatment of HCC. Two studies using the endothelial marker CD34 to identify neovascularisation have reported that high microvessel density was a significant predictor to poor disease-free survival after resection (Ho et al, 2006; Wada et al, 2006; Poon et al, 2007). The mechanisms responsible for angiogenesis in HCC are not completely understood. However, one of the most important stimuli for the formation of new tumour vessels is the interaction of vascular endothelial growth factor (VEGF) with the VEGF receptors 1 and 2. Vascular endothelial growth factor promotes the growth, migration and morphogenesis of vascular endothelial cells and increases vascular permeability. Although well-differentiated HCC express higher levels of VEGF, it has also been shown that circulating VEGF can be detected in patients with more undifferentiated carcinomas (Torimura et al, 1998). Moreover, a correlation between VEGF expression and survival has been described (Poon et al, 2007). Other factors important for neoangiogenesis in HCC include fibroblast growth factor 2 (FGF2), angiogenin and angiopoietin.
Growth receptors
Tyrosine kinase receptors represent an attractive target for molecular therapy of HCC. The Ras–MAP kinase (MAPK) signal transduction pathway, as well as the phosphatidylinositol-3 (PI3K)–AKT kinase, are directly activated through the engagement of different growth factor receptors. A number of growth factor receptors are important in hepatocarcinogenesis such as the epidermal growth factor receptor (EGFR), the fibroblast growth factor receptor (FGFR), the hepatocyte growth factor receptor (HGFR), the stem cell growth factor receptor (c-kit), the platelet growth factor receptor (PDGFR) as well as the VEGF receptor. Engagement of these factors activates the Grb2/shc/SOS complex, which initiates the activation of the Ras–Raf–ERK1/2 MAPK signalling pathway and therefore ultimately leads to cell proliferation. A number of different small molecules, which are currently in clinical use or under early clinical or preclinical evaluation, block these pathways at different levels. There are a number of small molecules targeting TK activity of EGFR that are being evaluated for the treatment of HCC (Figure 1).
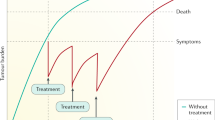
Sorafenib for the treatment of advanced HCC
The multi-tyrosine kinase inhibitor sorafenib is the first and so far the only drug that has shown overall survival benefit in patients with HCC in a multi-centre, double-blind, placebo-controlled randomised phase III trial (SHARP trial). Median overall survival increased from 7.9 months in the placebo group to 10.7 months in the sorafenib group (hazard ratio in the sorafenib group, 0.69; 95% confidence interval, 0.55–0.87; P<0.001) (Llovet et al, 2008b). Sorafenib blocks the Ras–Raf kinase pathway in the tumour cell as well as the VEGF and PDGF receptor on the endothelial cells (Wilhelm et al, 2004). Although a number of studies suggest the relevance of the Ras–Raf pathway in primary liver tumours, surprisingly, only one study detected BRAF mutations in liver tumours. However, these tumours were primary bile duct tumours (Tannapfel et al, 2003) suggesting that a major mode of action in the treatment of HCC is the potent antiangiogenic potential of sorafenib. The most common grade 3 drug-related adverse events observed in the SHARP study included diarrhoea and hand-foot skin reaction, both of which occurred in 8% of all patients treated with sorafenib. Recently, a survival benefit has also been shown for Asian patients with advanced HCC treated with sorafenib. In this study, the median overall survival increased from 4.1 months in the placebo group to 6.2 months in the sorafenib group (hazard ratio in the sorafenib group 0.67; 95% confidence interval, 0.49–0.93; P<0.0155) (Cheng et al, 2008). Based on these two pivotal studies sorafenib has become the new standard of care for patients with advanced HCC. Future studies will define the role of sorafenib also in the adjuvant setting or in combination with transarterial chemoembolisation. Although until today no data is available on the combination of sorafenib with other molecular targeting agents for the treatment of HCC, one phase II study using the combination of sorafenib and doxorubicin has been performed. This study while showing good efficacy also revealed significant doxorubicin-related toxicities (Abou-Alfa et al, 2008) (Table 1).
Results from phase I and II studies testing other approved targeting drugs
Sunitinib represents another small molecule that is currently being evaluated in a phase III trial for the treatment of HCC. Sunitinib is a multi-tyrosine kinase inhibitor that has already been approved for the treatment of renal cell carcinoma as well as gastrointestinal stroma tumours. It targets receptor tyrosine kinases of the split-kinase domain family such as VEGFR-1 and -2, PDGFR-α and PDGFR-β, c-kit and FLT3 and the RET kinase (O’Farrell et al, 2003). A number of different preclinical models have shown an enhanced efficacy of tyrosine kinase inhibitors if the VEGF receptors on surrounding endothelial cells as well as the PDGF receptors on pericytes are blocked simultaneously. Results from different phase II studies in which patients with unresectable and metastasised HCC were treated with 37.5 mg sunitinib daily are shown in Table 1. This treatment was reasonably well tolerated and 9 out of 19 patients showed stable disease 12 weeks after initiation of treatment. A partial response was observed in one patient and a decrease in tumour permeability in a number of patients (Zhu et al, 2007a). In a second study in patients with unresectable HCC, patients were treated with 50 mg sunitinib daily. Although one patient showed a partial response in this study, the sunitinib dosage had to be reduced in 27% of the patients due to adverse effects. In five cases an acute decompensation of the liver cirrhosis was observed (Faivre et al, 2007).
Erlotinib at a dosage of 150 mg daily has been evaluated in two independent phase II trials in patients with advanced HCC. Although the objective tumour response rates observed were only minor (6% in one trial and no responses in the other trial), stable diseases were observed in 50 and 43% of the cases, respectively (Philip et al, 2005; Thomas et al, 2007a), with a PFS rate of 32 and 28% after 6 months and 24 weeks. Lapatinib, a different EGFR tyrosine kinase inhibitor, has shown promising results in a phase II trial with 2 out of 17 partial responses and a modest PFS of 2.3 months (Ramanathan et al, 2006) (Table 1). Moreover, gefitinib, an oral EGFR tyrosine kinase inhibitor has been tested in patients with advanced HCC as a single agent. However, the authors have concluded that gefitinib was not effective in patients with advanced HCC (O’Dwyer et al, 2006) (Table 1). Cetuximab, an EGFR blocking antibody, has been tested in multiple phase II trials. Zhu and colleagues reported a PFS of 1.4 months (Zhu et al, 2007b) and no objective responses. In our study we observed similar results with a TTP of 2 months (Grunwald et al, 2007) (Table 1).
Bevacizumab is a recombinant humanised antibody directed against VEGF. It has been approved in the United States by the FDA for the treatment of a number of different tumours including colorectal cancer, non-small-cell lung cancer and breast carcinoma. Partial responses have been observed in patients after bevacizumab monotherapy and disease stabilisation in 30% of treated patients has been documented (Siegel et al, 2008). Combination treatment of bevacizumab with EGFR targeting agents was shown to be safe and revealed promising initial results with a median time to progression of 9 months (Thomas et al, 2007b) (see Table 1). Overall, bevacizumab has shown promising results as a single agent as well as in combination with other targeted therapies and cytotoxic therapy (Zhu et al, 2006) supporting the idea of a vascular targeting therapy for the treatment of HCC.
Reports from clinical trials testing new molecular targeting drugs for the treatment of HCC
AZD2171 is another small molecule, which by blocking the tyrosine kinase activity of VEGFR, PDGFR and c-kit, has shown remarkable effects in the treatment of glioblastoma. It has also been tested in a phase II trial in patients with non-resectable HCC. However, 84% of the patients experienced grade 3 toxicities at a dosage of 45 mg (Alberts et al, 2007). It remains an open question why AZD2171 was so poorly tolerated in patients with HCC. One possible explanation could be the underlying liver cirrhosis, which can impair drug metabolism significantly.
PTK787 is a compound that inhibits VEGFR1, VEGFR2, VEGFR3, PDGFR-β, Flt-3 and kit. It was tested in a phase III trial in patients with advanced colorectal cancer. However, the results from that trial were negative. In a murine HCC model oral administration of PTK787 induced tumour cell apoptosis and reduced vessel formation (Liu et al, 2005). Induction of hypoxia through ligation of the hepatic artery enhanced this effect (Yang et al, 2006). PTK787 might therefore represent an interesting combination treatment with transarterial chemoembolisation. The maximal tolerated dose was determined to be 750 mg daily in a phase I study of 18 patients with HCC and disease stabilisation was observed in 9 patients (Koch et al, 2005).
ZD6474 is a new oral inhibitor of VEGF receptors that additionally targets the EGFR/HER1 receptor. This drug has shown significant antitumour activity in preclinical tumour models and experimental data also suggests that it might inhibit metastasis formation. It is currently being tested in a phase II trial in patients with HCC. A phase I/II study of TSU-68, an oral angiogenesis inhibitor, which targets VEGF, β-FGF and PDGF, has shown good tolerability of this drug and preliminary data suggest its antitumour activity in patients with HCC. Brivanib, an oral VEGFR and FGFR tyrosine kinase inhibitor, is currently being tested in a phase I and II study in HCC patients and a phase III trial is being planned.
Compounds in early development for the treatment of HCC
AZD6244 is a new MEK inhibitor, which is being evaluated for the treatment of HCC in preclinical models. The MEK inhibitor, PD0325901, which can be administered orally, has been evaluated in TGF-α transgenic mice in which liver cancers were induced by diethylnitrosamine treatment. PD0325901 led to a significantly smaller number of premalignant tumour cells in the treatment group. So far there is no data available on the effect of MEK inhibitors in patients with HCC.
The PI3K pathway is activated by a number of different growth factors and cytokines. PI3K activation leads to the expression of phosphoinositol triphosphate and activation of the AKT (PKB) kinase. AKT has multiple cellular targets, which suppress induction of apoptosis in the cell. One important substrate of the AKT kinase is the ‘mammalian target of rapamycin’ protein family (mTOR). The mTOR regulates the activation of p70 S6 kinase and the translational repressor proteins PHAS-1/4E-BP. These proteins control the cell cycle and have therefore an impact on cell proliferation. AKT activation is antagonised by the expression of the lipid phosphatase PTEN. In recent years, different molecules have been developed which target this pathway at various steps. The sphingosine receptor inhibitor FTY720 controls PI3K activity by inhibition of rac proteins. Treatment of HCC cells with FTY720 results in reduced cell motility, which might affect metastasis formation (Lee et al, 2005). The macrolid antibiotic rapamycin binds cytoplasmic FK506 binding protein (FKBP12) thereby inhibiting mTOR activity. This might be one reason why, sirolimus as well as its derivates block growth of tumours with an activated PI3K–AKT signal transduction cascade. The activity of PTEN is reduced in more than 50% of all HCC. In addition, hepatocyte specific knockout of PTEN promotes hepatocarcinogenesis in mice. Mammalian target of rapamycin inhibitors such as sirolimus suppress the proliferation of hepatoma cells in vitro as well as in vivo in a xenotransplant model. Interestingly, the survival benefit observed in mice correlated with a decreased vascularisation of tumours in mice. Different mTOR inhibitors such as temsirolimus and RAD001 are currently being evaluated in preclinical models of HCC and first phase I/II trials have been initiated.
A different targeting pathway includes Wnt proteins that function as ligands for the so-called Frizzled family of G-protein coupled receptors. β-catenin is activated by the Wnt signal transduction pathway and binds the transcription factor TCF (T-cell factor) to initiate expression of a number of genes, which are important for proliferation and cell survival including cyclin D1, c-myc and others. Disturbing the Wnt signal transduction cascade at different levels can cause a constitutive activation of this pathway, which promotes hepatocarcinogenesis. Indeed, activating mutations in the Wnt pathway have been observed in up to 40% of all HCC. It should be noted at this point that Wnt pathway also plays an important role in liver regeneration and proliferation of stem cells opening the possibility to potentially inhibit the proliferation of tumour stem cells. Although no molecules targeting this pathway have made their way into clinical evaluation, different drugs are currently in preclinical testing such as PKF115-584 and CGP049090.
Epigenetic modifications of the genome (mainly hypermethylation of CpG island and histone deacetylation) accumulate during hepatocarcinogenesis in chronically injured liver cells. It has been shown that a large number of tumour-suppressor genes are inactivated by epigenetic mechanisms in HCC. Success in epigenetic therapy (such as 5-aza-2-deoxycytidine and SAHA) has been achieved in both haematological malignancies and solid tumours. In HCC cell lines, chemosensitivity can be potentiated by epigenetic therapy. A multi-centre phase I/II trial on a novel histone deacetylase inhibitor, PXD-101, is currently underway in Hong Kong.
Conclusion
In contrast to haematological malignancies such as CML, no single oncogenic event can be accused for the development of HCC. Instead, a multitude of different signalling pathways are affected in liver cancer cells making it difficult to focus molecular treatments. The results of recent clinical trials and the advent of the first systemic treatment for HCC point towards a multi-targeted therapeutic approach to this disease. The demonstration of an increase in overall survival of less than 3 months with the use of sorafenib in patients with advanced HCC can only be the beginning of a new era in the treatment of HCC. More drugs potentially targeting alternative pathways need to be evaluated in combination with sorafenib. In addition, more drugs targeting similar molecules need to be evaluated compared with sorafenib as recently suggested (Llovet et al, 2008a). However, understanding the exact mechanisms involved in hepatocarcinogenesis remains the fundamental condition for the development of new and more potential drugs for the treatment of HCC.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abou-Alfa GK, Johnson P, Knox J, Davidenko I, Lacava J, Leung T, Mori A, Le Berre M, Voliotis D, Saltz L (2008) Final results from a phase II (PhII), randomized, double-blind study of sorafenib plus doxorubicin (S+D) versus placebo plus doxorubicin (P+D) in patients (pts) with advanced hepatocellular carcinoma. In 2008 Gastrointestinal Cancers Symposium Abstract 128. American Society of Oncology, 25–27 January 2008, Orlando, FL, USA, p132
Alberts SR, Morlan BW, Kim GP, Pitot HC, Quevedo FJ, Dakhil SR, Gross HM, Merchan JR, Roberts RL (2007) NCCTG phase II trial (N044J) of AZD2171 for patients with hepatocellular carcinoma (HCC) – Interim review of toxicity. In Gastrointestinal Cancers Symposium Abstract 186. American Society of Oncology, 19–21 January 2007, Orlando, FL, USA, p178
Cheng A, Kang Y, Chen Z, Tsao C, Qin S, Kim J, Burock K, Zou J, Voliotis D, Guan ZZ (2008) Randomized phase III trial of sorafenib versus placebo in Asian patients with advanced hepatocellular carcinoma. J Clin Oncol 26: 4509
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132: 2557–2576
Faivre SJ, Raymond E, Douillard J, Boucher E, Lim HJ, Kim JS, Lanzalone S, Lechuga MJ, Sherman L, Cheng A (2007) Assessment of safety and drug-induced tumor necrosis with sunitinib in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 25: 3546
Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, Klempnauer J, Galanski M, Manns MP (2005) Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 92: 1862–1868
Grunwald V, Wilkens L, Gebel M, Greten TF, Kubicka S, Ganser A, Manns MP, Malek N (2007) A phase II open-label study of cetuximab in unresectable hepatocellular carcinoma: Final results. J Clin Oncol 25: 4598
Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, Poon RT (2006) Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology 44: 836–843
Hoda D, Catherine C, Strosberg J, Valone T, Jump H, Campos T, Halina G, Wood G, Hoffe S, Garrett CR (2008) Phase II study of sunitinib malate in adult pts (pts) with metastatic or surgically unresectable hepatocellular carcinoma (HCC). In 2008 Gastrointestinal Cancers Symposium Abstract 267. American Society of Oncology, 25–27 January 2008, Orlando, FL, USA, p202
Hsu C, Yang T, Hsu C, Toh H, Epstein RJ, Hsiao L, Cheng A (2007) Modified-dose capecitabine+bevacizumab for the treatment of advanced/metastatic hepatocellular carcinoma (HCC): a phase II, single-arm study. J Clin Oncol 25: 15190
Koch I, Baron A, Roberts S, Junker U, Palacay-Ramona M, Masson E, Kay A, Wiedenmann B, Laurent D, Cebon J (2005) Influence of Hepatic Dysfunction on Safety, Tolerability, and Pharmacokinetics (PK) of PTK787/ZK 222584 in Patients with Unresectable Hepatocellular Carcinoma. J Clin Oncol 23: 4134
Lee TK, Man K, Ho JW, Wang XH, Poon RT, Xu Y, Ng KT, Chu AC, Sun CK, Ng IO, Sun HC, Tang ZY, Xu R, Fan ST (2005) FTY720: a promising agent for treatment of metastatic hepatocellular carcinoma. Clin Cancer Res 11: 8458–8466
Liu Y, Poon RT, Li Q, Kok TW, Lau C, Fan ST (2005) Both antiangiogenesis- and angiogenesis-independent effects are responsible for hepatocellular carcinoma growth arrest by tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res 65: 3691–3699
Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ (2008a) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100 (10): 698–711
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008b) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390
Louafi S, Hebbar M, Rosmorduc O, Tesmoingt C, Asnacios A, Romano O, Fartoux L, Artru P, Poynard T, Taieb J (2007) Gemcitabine, oxaliplatin (GEMOX) and cetuximab for treatment of hepatocellular carcinoma (HCC): results of the phase II study ERGO. J Clin Oncol 25: 4594
Malka D, Dromain C, Farace F, Horn D, Pignon J, Ducreux M, Boige V (2007) Bevacizumab in patients (pts) with advanced hepatocellular carcinoma (HCC): preliminary results of a phase II study with circulating endothelial cell (CEC) monitoring. J Clin Oncol 25: 4570
O’Dwyer PJ, Giantonio BJ, Levy DE, Kauh JS, Fitzgerald DB, Benson AB (2006) Gefitinib in advanced unresectable hepatocellular carcinoma: Results from the Eastern Cooperative Oncology Group's Study E1203. J Clin Oncol 24: 4143
O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM (2003) SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101: 3597–3605
O’Neil BH, Bernard SA, Goldberg RM, Moore DT, Garcia R, Marroquin C, Morse MA, Woods L, Sanoff HK (2008) Phase II study of oxaliplatin, capecitabine, and cetuximab in advanced hepatocellular carcinoma. J Clin Oncol 26: 4604
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C (2005) Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 23: 6657–6663
Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST (2007) High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol 14: 1835–1845
Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, Kindler HL, Iqbal S, Longmate J, Gandara DR (2006) Phase II study of lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase 1 and 2 (Her2/Neu) in patients (pts) with advanced biliary tree cancer (BTC) or hepatocellular cancer (HCC). A California Consortium (CCC-P) Trial. J Clin Oncol 24: 4010
Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J, Christos P, Mazumdar M, Popa E, Brown Jr RS, Rafii S, Schwartz JD (2008) Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 26: 2992–2998
Sun W, Haller DG, Mykulowycz K, Rosen M, Soulen M, Capparo M, Faust T, Giantonia B, Olthoff K (2007) Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): a phase II study. J Clin Oncol 25: 4574
Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, Hauss J, Wittekind C (2003) Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 52: 706–712
Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, Rashid A, Dancey J, Abbruzzese JL (2007a) Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer 110: 1059–1067
Thomas MB, Chadha R, Iwasaki M, Glover K, Abbruzzese JL (2007b) The combination of bevacizumab and erlotinib shows significant biological activity in patients with advanced hepatocellular carcinoma. J Clin Oncol 25: 4567
Torimura T, Sata M, Ueno T, Kin M, Tsuji R, Suzaku K, Hashimoto O, Sugawara H, Tanikawa K (1998) Increased expression of vascular endothelial growth factor is associated with tumor progression in hepatocellular carcinoma. Hum Pathol 29: 986–991
Wada H, Nagano H, Yamamoto H, Yang Y, Kondo M, Ota H, Nakamura M, Yoshioka S, Kato H, Damdinsuren B, Tang D, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Nakamori S, Sakon M, Dono K, Wakasa K, Monden M (2006) Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int 26: 414–423
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099–7109
Yang ZF, Poon RT, Liu Y, Lau CK, Ho DW, Tam KH, Lam CT, Fan ST (2006) High doses of tyrosine kinase inhibitor PTK787 enhance the efficacy of ischemic hypoxia for the treatment of hepatocellular carcinoma: dual effects on cancer cell and angiogenesis. Mol Cancer Ther 5: 2261–2270
Zhu AX, Blaszkowsky LS, Ryan DP, Clark JW, Muzikansky A, Horgan K, Sheehan S, Hale KE, Enzinger PC, Bhargava P, Stuart K (2006) Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 1898–1903
Zhu AX, Sahani DV, di Tomaso E, Duda D, Sindhwani V, Yoon SS, Blaszkowsky LS, Clark JW, Ryan DP, Jain RK (2007a) A phase II study of sunitinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 25: 4637
Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, Enzinger PC, Bhargava P, Meyerhardt JA, Horgan K, Fuchs CS, Ryan DP (2007b) Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 110 (3): 581–589
Acknowledgements
The authors are supported by funds from the German Research Foundation DFG (KFO 119), grants from the Deutsche Krebshilfe (Max-Eder Program) to NM, DFG cluster of excellence rebirth to NM and Helmholtz Alliance to TG and MM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Greten, T., Korangy, F., Manns, M. et al. Molecular therapy for the treatment of hepatocellular carcinoma. Br J Cancer 100, 19–23 (2009). https://doi.org/10.1038/sj.bjc.6604784
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604784
Keywords
This article is cited by
-
Therapeutic options in hepatocellular carcinoma: a comprehensive review
Clinical and Experimental Medicine (2023)
-
Tackling hepatitis B virus-associated hepatocellular carcinoma—the future is now
Cancer and Metastasis Reviews (2013)
-
Cryotherapy is Associated with Improved Clinical Outcomes of Sorafenib Therapy for Advanced Hepatocellular Carcinoma
Cell Biochemistry and Biophysics (2012)
-
A hepatoprotective Lindera obtusiloba extract suppresses growth and attenuates insulin like growth factor-1 receptor signaling and NF-kappaB activity in human liver cancer cell lines
BMC Complementary and Alternative Medicine (2011)
-
MicroRNA122 is a key regulator of α-fetoprotein expression and influences the aggressiveness of hepatocellular carcinoma
Nature Communications (2011)