Abstract
Mothers with a CYP2D6 ultrarapid metabolizer phenotype may expose their infants to risk of adverse events when taking codeine while breastfeeding, by producing more of the active metabolite, morphine. Pharmacogenetic testing may be a valuable tool to identify such mothers, but testing can be costly. The objective of the study was to determine the incremental costs of genotyping to avert neonatal adverse events during maternal pharmacotherapy. A cost-effectiveness analysis, using a decision model, was performed with a hypothetical cohort of prenatal subjects. Parameter estimates, costs and ranges for sensitivity analyses were ascertained from the literature and expert opinion. Sensitivity analyses were conducted to assess the robustness of the results. Probabilistic sensitivity analysis revealed an incremental cost-effectiveness (ICER) of $10 433 (Canadian dollars) for genotyping compared to no genotyping per adverse event averted. Results were sensitive to hospital admission costs. The ICER was lower when evaluating only subjects having caesarean deliveries or those from ethnic populations known to have a high prevalence of ultra-rapid metabolizers. Although genotyping to guide pharmacotherapy was not cost saving, the cost to avert an infant adverse event may represent good value for money in specific populations. With a growing demand for personalized medicine, these findings are relevant for decision makers, clinicians and patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
World Health Organization Report of the Expert Consultation on the Optimal Duration of Exclusive Breastfeeding. Department of Nutrition for Health and Development and Department of Child and Adolescent Health and Development: Geneva, Switzerland, 2001.
Critch JN . Nutrition for healthy term infants, birth to six months: An overview. Paediatr Child Health 2013; 18: 206–209.
Johnson M, Landers S, Noble L, Szucs K, Viehmann L . The American Academy of Pediatrics. breastfeeding and the use of human milk. Pediatrics 2012; 129: e827–e841.
American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001; 108: 776–789.
Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ . Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006; 368: 704.
US Food and Drug Administration 2007, Use of codeine by some breastfeeding mothers may lead to life-threatening side effects in nursing babies. Accessed June 1, 2014, from http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm054717.htm.
Health Canada Marketed Health Products Directorate 2008, Important Safety Information on Tylenol with Codeine in Nursing Mothers and Ultra-Rapid Metabolizers of Codeine. Accessed June 1, 2014, from http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2008/13255a-eng.php.
European Medicines Agency 2013, Assessment report for codeine-containing medicinal products indicated in the management of pain in children. London. EMA/441891/2013.
Madadi P, Ross CJ, Hayden MR, Carleton BC, Gaedigk A, Leeder JS et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin Pharmacol Ther 2009; 85: 31–35.
Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 2014; 95: 376–382.
Wadelius M, Darj E, Frenne G, Rane A . Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther 1997; 62: 400–407.
Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjoqvist F, Ingelman-Sundberg M . Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci USA 1993; 90: 11825–11829.
Llerena A, Naranjo ME, Rodrigues-Soares F, Penas-LLedo EM, Farinas H, Tarazona-Santos E . Interethnic variability of CYP2D6 alleles and of predicted and measured metabolic phenotypes across world populations. Expert Opin Drug Metab Toxicol 2014; 10: 1569–1583.
Shah RR, Gaedigk A, Llerena A, Eichelbaum M, Stingl J, Smith RL . CYP450 genotype and pharmacogenetic association studies: a critical appraisal. Pharmacogenomics 2016; 17: 259–275.
Gaedigk A . Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry 2013; 25: 534–553.
Sistonen J, Sajantila A, Lao O, Corander J, Barbujani G, Fuselli S . CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics 2007; 17: 93–101.
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C . Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 2007; 116: 496–526.
Levy S, Sutton G, Ng PC, Feuk L, Halpern AL, Walenz BP et al. The diploid genome sequence of an individual human. PLoS Biol 2007; 5: e254.
Vegter S, Boersma C, Rozenbaum M, Wilffert B, Navis G, Postma MJ . Pharmacoeconomic evaluations of pharmacogenetic and genomic screening programmes: a systematic review on content and adherence to guidelines. Pharmacoeconomics 2008; 26: 569–587.
Beaulieu M, de Denus S, Lachaine J . Systematic review of pharmacoeconomic studies of pharmacogenomic tests. Pharmacogenomics 2010; 11: 1573–1590.
Wong WB, Carlson JJ, Thariani R, Veenstra DL . Cost effectiveness of pharmacogenomics: a critical and systematic review. Pharmacoeconomics 2010; 28: 1001–1013.
Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Canadian Agency for Drugs and Technologies in Health: Ottawa. Ontario, Canada, 2006.
TreeAge Software Inc TreeAge Pro [computer program] Build 16.2. TreeAge Software, Inc: Williamstown, MA, USA, 2016.
Kelly LE, Chaudhry SA, Rieder MJ, 't Jong G, Moretti ME, Lausman A et al. A clinical tool for reducing central nervous system depression among neonates exposed to codeine through breast milk. PLoS One 2013; 8: e70073.
Moretti ME . A Cost-effectiveness Analysis of Maternal Genotyping to Guide Treatment for Postpartum Pain And Avert Inrant Adverse Events. Institute of Health Policy Management and Evaluation, University of Toronto, Ontario, Canada, 2014.
Mkontwana N, Novikova N . Oral analgesia for relieving post-caesarean pain. Cochrane Database Syst Rev 2015: CD010450.
Deussen AR, Ashwood P, Martis R . Analgesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev 2011: CD004908.
Davis JM, Bhutani VK . Neonatal apnea and maternal codeine use. Pediatr Res 1985; 19: 170A.
Meny RG, Naumburg EG, Alger LS, Brill-Miller JL, Brown S . Codeine and the breastfed neonate. J Hum Lact 1993; 9: 237–240.
Findlay JW, DeAngelis RL, Kearney MF, Welch RM, Findlay JM . Analgesic drugs in breast milk and plasma. Clin Pharmacol Ther 1981; 29: 625–633.
Naumburg EG, Meny RG . Breast milk opioids and neonatal apnea. Am J Dis Child 1988; 142: 11–12.
Ito S, Blajchman A, Stephenson M, Eliopoulos C, Koren G . Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am J Obstet Gynecol 1993; 168: 1393–1399.
Anderson PO, Pochop SL, Manoguerra AS . Adverse drug reactions in breastfed infants: less than imagined. Clin Pediatr 2003; 42: 325–340.
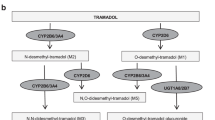
Thorn CF, Klein TE, Altman RB . Codeine and morphine pathway. Pharmacogenet Genomics 2009; 19: 556–558.
Lotsch J, Skarke C, Liefhold J, Geisslinger G . Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet 2004; 43: 983–1013.
Ungar WJ . Challenges in health state valuation in paediatric economic evaluation: are QALYs contraindicated? Pharmacoeconomics 2011; 29: 641–652.
Kohlrausch FB, Gama CS, Lobato MI, Belmonte-de-Abreu P, Gesteira A, Barros F et al. Molecular diversity at the CYP2D6 locus in healthy and schizophrenic southern Brazilians. Pharmacogenomics 2009; 10: 1457–1466.
Cascorbi I . Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest 2003; 33 (Suppl 2): 17–22.
Almoguera B, Riveiro-Alvarez R, Gomez-Dominguez B, Lopez-Rodriguez R, Dorado P, Vaquero-Lorenzo C et al. Evaluating a newly developed pharmacogenetic array: screening in a Spanish population. Pharmacogenomics 2010; 11: 1619–1625.
Fleeman N, Dundar Y, Dickson R, Jorgensen A, Pushpakom S, McLeod C et al. Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: systematic review and meta-analyses. Pharmacogenomics J 2011; 11: 1–14.
Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y et al. The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation. Health Technol Assess 2010; 14: 1–157, iii.
East N, Dube J, Perreault EL . Postpartum pain relief: a randomized comparison of self-administered medication and standard administration. J Obstet Gynaecol Can 2007; 29: 975–981.
Lam J, Kelly L, Ciszkowski C, Landsmeer ML, Nauta M, Carleton BC et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr 2012; 160: 33–37.
Ministry of Health and Long Term Care2013 Schedule of Benefits - Physician Services Under the Health Insurance Act. Ministry of Health and Long-Term Care: Toronto, Ontario, Canada, 2013..
Ontario Ministry of Health and Long-Term Care Ontario Drug Benefit Formulary/comparative Drug Index. 42nd edn. Ministry of Health and Long-Term Care: Toronto, Ontario, Canada, 2014.
Hancock RL, Ungar WJ, Einarson A, Goodstadt M, Koren G . Providing information regarding exposures in pregnancy: a survey of North American Teratology Information Services. Reprod Toxicol 2008; 25: 381–387.
Ontario Ministry of Health and Long-Term Care 2012, Ambulance Services Billing..
Ministry of Health and Long Term Care HDBDSU 2009, Ontario Case Costing Guide Version 7.0..
Statistics Canada 2014, Table 282-0073 - Labour force survey estimates (LFS), wages of employees by job permanence, union coverage, sex and age group, unadjusted for seasonality, monthly, CANSIM..
Statistics Canada 2014., Table 282-0069 - Labour force survey estimates (LFS), wages of employees by type of work, National Occupational Classification for Statistics, sex and age group, unadjsuted for seasonality, monthly, CANSIM..
Acknowledgements
This work was supported by scholarships from the Ontario Student Opportunity Trust Fund – Hospital for Sick Children Foundation Student Scholarship Program, the Ontario Graduate Scholarship Program, the Institute of Health Policy, Management and Evaluation and the Canadian Federation of University Women.
Author contributions
MEM, HB, GK, SI, WJU designed the research. MEM, DFL performed the research and analyzed the data. MEM, WJU wrote the paper. MEM, DFL performed the research and analyzed the data. MEM, WJU wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Moretti, M., Lato, D., Berger, H. et al. A cost-effectiveness analysis of maternal CYP2D6 genetic testing to guide treatment for postpartum pain and avert infant adverse events. Pharmacogenomics J 18, 391–397 (2018). https://doi.org/10.1038/tpj.2017.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2017.33
This article is cited by
-
Progress, Challenges, and Prospects of Research on the Effect of Gene Polymorphisms on Adverse Reactions to Opioids
Pain and Therapy (2022)
-
Application of pharmacogenetics in clinical practice: problems and solutions
Journal of Neural Transmission (2019)
-
Cost-effectiveness of precision medicine: a scoping review
International Journal of Public Health (2019)