Abstract
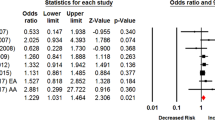
Many studies have examined the association between SLC6A3 3′-untranslated region (UTR) variable number tandem repeat (VNTR) polymorphism and smoking cessation; however, the results are inconclusive, primarily because of the small-to-moderate size samples. The primary goal of this study was to determine whether this polymorphism has any effect on smoking cessation by a meta-analysis of all reported studies. We adopted a 9-repeat dominant model that considers 9-repeat and non-9-repeat as two genotypes and compared their frequencies in former vs current smokers. Eleven studies with 5480 participants were included. Considering the presence of study heterogeneity and differences in the availability of information from each study, three separate meta-analyses were performed with the Comprehensive Meta-Analysis statistical software (version 2.0). The first meta-analysis provided evidence of association between the 9-repeat genotype and smoking cessation under the fixed-effects model (pooled odds ratio (OR)=1.13; 95% confidence interval (CI)=1.01, 1.27; P=0.037) but not in the random-effects model (pooled OR=1.11; 95% CI=0.96, 1.29; P=0.159). Given the marginal evidence of heterogeneity among studies (P=0.10; I2=35.9%), which likely was caused by inclusion of an Asian population treatment study with an opposite effect of the polymorphism on smoking cessation, we excluded the data of this study, revealing a significant association between the 9-repeat genotype and smoking cessation under both the fixed- and random-effects models (pooled OR=1.15; 95% CI=1.02, 1.29; P=0.02 for both models). By analyzing adjusted and unadjusted results, we performed the third meta-analysis, which showed consistently that the 9-repeat genotype was significantly associated with smoking cessation under both the fixed- and random-effects models (pooled OR=1.17; 95% CI=1.04, 1.31; P=0.009 for both models). We conclude that the 3′-UTR VNTR polymorphism is significantly associated with smoking cessation, and smokers with one or more 9-repeat alleles have a 17% higher probability of smoking cessation than smokers carrying no such allele.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
WHO. WHO Tobacco Fact sheet N°339 http://www.who.int/mediacentre/factsheets/fs339/en/ World Health Organization 2014.
Carmelli D, Swan GE, Robinette D, Fabsitz R . Genetic influence on smoking—a study of male twins. N Engl J Med 1992; 327: 829–833.
Heath AC, Martin NG . Genetic models for the natural history of smoking: evidence for a genetic influence on smoking persistence. Addict Behav 1993; 18: 19–34.
Heath AC, Cates R, Martin NG, Meyer J, Hewitt JK, Neale MC et al. Genetic contribution to risk of smoking initiation: comparisons across birth cohorts and across cultures. J Subst Abuse 1993; 5: 221–246.
True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N et al. Genetic and environmental contributions to smoking. Addiction 1997; 92: 1277–1287.
Li MD, Cheng R, Ma JZ, Swan GE . A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 2003; 98: 23–31.
Sullivan PF, Kendler KS . The genetic epidemiology of smoking. Nicotine Tob Res 1999; 1 (Suppl 2): S51–S57.
Xian H, Scherrer JF, Madden PA, Lyons MJ, Tsuang M, True WR et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res 2003; 5: 245–254.
Hardie TL, Moss HB, Lynch KG . Genetic correlations between smoking initiation and smoking behaviors in a twin sample. Addict Behav 2006; 31: 2030–2037.
David SP, Munafo MR . Genetic variation in the dopamine pathway and smoking cessation. Pharmacogenomics 2008; 9: 1307–1321.
Li MD . The genetics of nicotine dependence. Curr Psychiatry Rep 2006; 8: 158–164.
Ma Y, Yuan W, Jiang X, Cui WY, Li MD . Updated Findings of the Association and Functional Studies of DRD2/ANKK1 Variants with Addictions. Mol Neurobiol 2014; 51: 281–299.
Wang J, Li MD . Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology 2010; 35: 702–719.
Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics 1992; 14: 1104–1106.
Dani JA, Heinemann S . Molecular and cellular aspects of nicotine abuse. Neuron 1996; 16: 905–908.
Caron MG . Images in neuroscience. Molecular biology, II. A dopamine transporter mouse knockout. Am J Psychiatry 1996; 153: 1515.
Uhl GR . Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Mov Disord 2003; 18 (Suppl 7): S71–S80.
Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol 1999; 18: 14–20.
Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S et al. A genetic association for cigarette smoking behavior. Health Psychol 1999; 18: 7–13.
Styn MA, Nukui T, Romkes M, Perkins K, Land SR, Weissfeld JL . The impact of genetic variation in DRD2 and SLC6A3 on smoking cessation in a cohort of participants 1 year after enrollment in a lung cancer screening study. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 254–261.
Hiemstra M, Engels RC, Barker ED, van Schayck OC, Otten R . Smoking-specific parenting and smoking onset in adolescence: the role of genes from the dopaminergic system (DRD2, DRD4, DAT1 genotypes). PLoS ONE 2013; 8: e61673.
Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B et al. Association of smoking and personality with a polymorphism of the dopamine transporter gene: results from a community survey. Am J Med Genet 2000; 96: 331–334.
Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O'Connor R, Stauffer RL et al. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different. Nicotine Tob Res 2002; 4: 333–340.
Sieminska A, Buczkowski K, Jassem E, Niedoszytko M, Tkacz E . Influences of polymorphic variants of DRD2 and SLC6A3 genes, and their combinations on smoking in Polish population. BMC Med Genet 2009; 10: 92.
Gordiev M, Engstrom PF, Khasanov R, Moroshek A, Sitdikov R, Dgavoronkov V et al. Genetic analysis of polymorphisms in dopamine receptor and transporter genes for association with smoking among cancer patients. Eur Addict Res 2013; 19: 105–111.
van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nuclear Med 2005; 46: 745–751.
Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB et al. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology 2001; 24: 553–560.
Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000; 22: 133–139.
Brookes KJ, Neale BM, Sugden K, Khan N, Asherson P, D'Souza UM . Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. Am J Med Genet B Neuropsychiatr Genet 2007; 144B: 1070–1078.
Mill J, Asherson P, Browes C, D'Souza U, Craig I . Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet 2002; 114: 975–979.
Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S . The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J 2001; 1: 152–156.
Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychol 2007; 26: 361–368.
O'Gara C, Stapleton J, Sutherland G, Guindalini C, Neale B, Breen G et al. Dopamine transporter polymorphisms are associated with short-term response to smoking cessation treatment. Pharmacogenet Genomics 2007; 17: 61–67.
Han DH, Joe KH, Na C, Lee YS . Effect of genetic polymorphisms on smoking cessation: a trial of bupropion in Korean male smokers. Psychiatric genetics 2008; 18: 11–16.
Munafo M, Clark T, Johnstone E, Murphy M, Walton R . The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res 2004; 6: 583–597.
Stapleton JA, Sutherland G, O'Gara C . Association between dopamine transporter genotypes and smoking cessation: a meta-analysis. Addict Biol 2007; 12: 221–226.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012.
David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafo MR et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction 2013; 108: 2202–2211.
Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr., Pinto A et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol 2003; 22: 541–548.
David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafo MR et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine Tob Res 2007; 9: 821–833.
Tashkin DP, Rabinoff M, Noble EP, Ritchie TL, Simmons MS, Connett J . Association of dopamine-related gene alleles, smoking behavior and decline in FEV1 in subjects with COPD: findings from the lung health study. Copd 2012; 9: 620–628.
Ton TG, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM . Genetic polymorphisms in dopamine-related genes and smoking cessation in women: a prospective cohort study. Behav Brain Funct 2007; 3: 22.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Wang F, Simen A, Arias A, Lu QW, Zhang H . A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Hum Genet 2013; 132: 347–358.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Egger M, Davey Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Hou QF, Li SB . Potential association of DRD2 and DAT1 genetic variation with heroin dependence. Neurosci Lett 2009; 464: 127–130.
Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T et al. Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism. Mol Psychiatry 1999; 4: 552–557.
Chen WJ, Chen CH, Huang J, Hsu YP, Seow SV, Chen CC et al. Genetic polymorphisms of the promoter region of dopamine D2 receptor and dopamine transporter genes and alcoholism among four aboriginal groups and Han Chinese in Taiwan. Psychiatr Genet 2001; 11: 187–195.
Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jablonski M, Rommelspacher H, Samochowiec A et al. Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neurosci Lett 2006; 410: 1–5.
Cook EH Jr., Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet 1995; 56: 993–998.
Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol psychiatry 2005; 10: 686–698.
Rommelse NN, Altink ME, Arias-Vasquez A, Buschgens CJ, Fliers E, Faraone SV et al. A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 1536–1546.
Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M . Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry 1997; 2: 311–313.
Greenwood TA, Kelsoe JR . Promoter and intronic variants affect the transcriptional regulation of the human dopamine transporter gene. Genomics 2003; 82: 511–520.
Kelada SN, Costa-Mallen P, Checkoway H, Carlson CS, Weller TS, Swanson PD et al. Dopamine transporter (SLC6A3) 5' region haplotypes significantly affect transcriptional activity in vitro but are not associated with Parkinson's disease. Pharmacogenet Genomics 2005; 15: 659–668.
Bergen AW, Conti DV, Van Den Berg D, Lee W, Liu J, Li D et al. Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology 2009; 34: 2252–2264.
Noble EP St, Jeor ST, Ritchie T, Syndulko K St, Jeor SC, Fitch RJ et al. D2 dopamine receptor gene and cigarette smoking: a reward gene? Med Hypotheses 1994; 42: 257–260.
Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D . The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics 1996; 6: 73–79.
Li MD, Ma JZ, Beuten J . Progress in searching for susceptibility loci and genes for smoking-related behaviour. Clin Genet 2004; 66: 382–392.
Yoshida K, Hamajima N, Kozaki K, Saito H, Maeno K, Sugiura T et al. Association between the dopamine D2 receptor A2/A2 genotype and smoking behavior in the Japanese. Cancer Epidemiol Biomarkers Prev 2001; 10: 403–405.
Hamajima N, Ito H, Matsuo K, Saito T, Tajima K, Ando M et al. Association between smoking habits and dopamine receptor D2 taqI A A2 allele in Japanese males: a confirmatory study. J Epidemiol 2002; 12: 297–304.
Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 2006; 59: 966–974.
Dluzen DE, Anderson LI . Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett 1997; 230: 140–142.
Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT . Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res 2006; 8: 627–638.
Acknowledgements
We thank Dr David L Bronson for excellent editing of this manuscript. We also thank Dr Sean P David of Stanford University for providing the original genotyping data reported in his paper (David et al., 2007) to us. This study was supported in part by the Research Center for Air Pollution and Health of Zhejiang University, Ministry of Science and Technology of China (2012AA020405), the National Natural Science Foundation of China grant 81273223, the Young Scientists Fund of National Science Foundation of China (81301140) and the NIH grant DA012844.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, Y., Yuan, W., Cui, W. et al. Meta-analysis reveals significant association of 3′-UTR VNTR in SLC6A3 with smoking cessation in Caucasian populations. Pharmacogenomics J 16, 10–17 (2016). https://doi.org/10.1038/tpj.2015.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.44
This article is cited by
-
Meta-analytic method reveal a significant association of theBDNF Val66Met variant with smoking persistence based on a large samples
The Pharmacogenomics Journal (2020)
-
Significant association of the CHRNB3-CHRNA6 gene cluster with nicotine dependence in the Chinese Han population
Scientific Reports (2017)
-
Association of STAT4 polymorphisms with hepatitis B virus infection and clearance in Chinese Han population
Amino Acids (2016)
-
The significant association of Taq1A genotypes in DRD2/ANKK1 with smoking cessation in a large-scale meta-analysis of Caucasian populations
Translational Psychiatry (2015)