Abstract
Major depressive disorder (MDD), common in the elderly, is a risk factor for dementia. Abnormalities in glutamatergic neurotransmission via the N-methyl-d-aspartate receptor (NMDA-R) have a key role in the pathophysiology of depression. This study examined whether depression was associated with cerebrospinal fluid (CSF) levels of NMDA-R neurotransmission-associated amino acids in cognitively intact elderly individuals with MDD and age- and gender-matched healthy controls. CSF was obtained from 47 volunteers (MDD group, N=28; age- and gender-matched comparison group, N=19) at baseline and 3-year follow-up (MDD group, N=19; comparison group, N=17). CSF levels of glutamine, glutamate, glycine, l-serine and d-serine were measured by high-performance liquid chromatography. CSF levels of amino acids did not differ across MDD and comparison groups. However, the ratio of glutamine to glutamate was significantly higher at baseline in subjects with MDD than in controls. The ratio decreased in individuals with MDD over the 3-year follow-up, and this decrease correlated with a decrease in the severity of depression. No correlations between absolute amino-acid levels and clinical variables were observed, nor were correlations between amino acids and other biomarkers (for example, amyloid-β42, amyloid-β40, and total and phosphorylated tau protein) detected. These results suggest that abnormalities in the glutamine–glutamate cycle in the communication between glia and neurons may have a role in the pathophysiology of depression in the elderly. Furthermore, the glutamine/glutamate ratio in CSF may be a state biomarker for depression.
Similar content being viewed by others
Introduction
Late-life depression, one of the most common psychiatric disorders in older adults, is associated with significant functional impairment, variable treatment response, high recurrence rates, chronicity and high rates of medical comorbidity and mortality.1, 2 Multiple lines of evidence suggest that late-life depression is a risk factor for the development of dementia, including Alzheimer’s disease (AD) and vascular dementia.2, 3, 4 However, the precise molecular mechanisms underlying the relationship between late-life depression and dementia risk remain unknown. Better understanding this relationship would likely contribute to improving preventive interventions in the elderly.
Glutamate (or l-glutamic acid) has an important role as the major excitatory neurotransmitter in the central nervous system. In the brain, glutamate is synthesized from glutamine by glutaminase, and metabolized to glutamine by glutamine synthetase (GS) in astrocytes.5, 6, 7, 8 Accumulating evidence suggests that abnormalities in the glutamatergic neurotransmission via the N-methyl-d-aspartate receptor (NMDA-R) have a key role in the pathophysiology of major depressive disorder (MDD).5, 6, 8, 9, 10, 11, 12 Hashimoto et al.13 reported increased levels of glutamate in the prefrontal cortex in postmortem brain samples from MDD and bipolar disorder, suggesting a role of the glutamatergic system in mood disorders. A noninvasive in vivo proton magnetic resonance spectroscopy (1H-MRS) study revealed increased levels of glutamate in the occipital cortex of patients with MDD,14 although other 1H-MRS studies found decreased levels of Glx (glutamate and glutamine) in the anterior cingulate cortex15 and dorsomedial/dorsal anterolateral prefrontal cortex16 in patients with MDD. In contrast, there was no difference in glutamate levels in the occipital cortex between MDD patients and controls.17 A recent MRS study showed that young MDD (N=90) and bipolar disorder (N=75) groups (18–30 years) did not show different levels of hippocampal glutamate compared with controls (N=40).18 A recent meta-analysis showed a significant reduction in Glx levels, but not glutamate levels alone, in the brains of depressed patients compared with controls across the studies included.19
Levine et al.20 reported higher cerebrospinal fluid (CSF) levels of glutamine in unmedicated patients with MDD. In contrast, a study showed low glutamate levels in the CSF of refractory patients with affective disorder.21 A recent study showed no significant differences between the control (N=25) and the MDD groups (N=18) in baseline CSF levels of glutamate (P=0.761) and glutamine (P=0.226).22 Thus, the results of studies on CSF levels of glutamine and glutamate in MDD patients are inconsistent. At present, there is no report that has evaluated the ratio of glutamine to glutamate in CSF of MDD patients although this ratio is an index of glutamine–glutamate cycle in glia–neuron communication.5, 6, 8, 23, 24 As the abnormalities in NMDA-R neurotransmission by altered glutamine–glutamate cycle in the brain may be involved in the pathophysiology of MDD,5, 6 it is of great interest to examine CSF levels of amino acids related with NMDA-R in elderly patients with MDD, and age- and gender-matched healthy controls.
In the present study, we measured CSF levels of amino acids (for example, glutamate, glutamine, glycine, d-serine and l-serine) in elderly patients with MDD, and age- and gender-matched healthy controls. All these amino acids can affect glutamatergic neurotransmission via the NMDA-R.13, 25 Furthermore, we also measured 3-year follow-up CSF samples in these subjects.
Materials and methods
Participants
This study was approved by the institutional review boards of the Nathan Kline Institute for Psychiatric Research and the New York University School of Medicine. Participants were volunteers who responded to advertisements in local newspapers and flyers, or were recruited from the Memory Education and Research Initiative Program.26 All participants provided informed consent before examination, and received up to $450.00 in compensation. A total of 133 participants completed the baseline evaluation, and 51 of these took part in the optional lumbar puncture procedure. Of these 51 participants, 3 were excluded because of evidence in their magnetic resonance imaging scans of confluent deep or periventricular white matter hyperintensities, defined as one or more hyperintense lesions measuring at least 10 mm in any direction. One individual was excluded because of a Mini-Mental State Examination (MMSE) score below 28. Of the 47 remaining participants, 28 were diagnosed with MDD by a board-certified psychiatrist, leaving 19 comparison subjects. The Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV disorders (SCID) was administered by a psychiatrist to establish an MDD diagnosis. Of the 28 individuals with MDD, 21 (75%) had recurrent episodes. Table 1 summarizes the demographic and clinical characteristics of the study participants at baseline.
Procedure
The study was conducted over four visits, usually 1 week apart. The first three visits were conducted at the Nathan Kline Institute for Psychiatric Research and the Clinical and Translational Science Institute, New York University Langone Medical Center. During the first visit, for the purpose of obtaining informed consent, study procedures were explained and participants were informed of their rights. Participants’ medical and psychiatric histories, including family history of AD, were also obtained, and their vital signs were measured. Participants then underwent a psychiatric evaluation, and their global cognitive status was assessed using the MMSE. In addition, the Hamilton Depression Rating Scale (HAM-D) was administered to rate the severity of current depressive symptoms. Subjects who met the criteria for past MDD but were not currently depressed (that is, HAM-D score below 16) were included as MDD subjects. Blood was drawn for routine medical testing and APOE genotyping. During the second visit, participants underwent a magnetic resonance imaging scan of the head to quantify the magnitude of vascular brain pathology. During the third visit, subjects underwent a comprehensive neuropsychological assessment, including the Buschke Selective Reminding Test,27 the Trail-Making Test parts A and B,28 and the category fluency test.29
Finally, during the fourth visit, a lumbar puncture was performed by a neuroradiologist under guided fluoroscopy in a subset of participants. Before the procedure, which was performed between 0900 and 1000 hours, participants were asked to fast overnight. A total of 15 ml of clear CSF was collected in three polypropylene tubes labeled ‘A’ (first 5 ml), ‘B’ (second 5 ml) and ‘C’ (third 5 ml). The tubes were immediately placed on ice for a maximum of 1 h until the samples were centrifuged at 4 °C (at 1500 r.p.m.) for 10 min. Then, aliquots of 0.25 ml were placed into 1.00-ml polypropylene cryogenic vials and put into Nunc eight-cell storage boxes (Nalge Nunc International, Rochester, NY, USA) at −80 °C. All amyloid-β, tau and amino-acid determinations were performed from tube ‘C’.
Among these participants, MDD patients (N=19) and comparison control subjects (N=17) were followed for 3 years. Fifteen individuals with MDD were receiving antidepressant treatment at the time of testing, but no differences in amyloid-β42 levels were observed within the MDD group as a function of antidepressant treatment.26 Clinical data, including physical examination, routine laboratory tests, psychiatric evaluations, HAM-D rating scale, cognitive functions and CSF samples, were collected at 3-year follow-up.
Measurement of amino acids
This study was also approved by the Research Ethics Committee of the Graduate School of Medicine, Chiba University. Measurement of CSF levels of amino acids was carried out using high-performance liquid chromatography (HPLC) system (Shimadzu, Kyoto, Japan) as previously reported.23, 25, 30 Briefly, 15 μl of H2O (HPLC grade), 20 μl of 0.1 m borate buffer (pH 8.0) and 60 μl of 50 mm NBD-F in CH3CN (HPLC grade) were added into 5 μl of CSF sample. The reaction mixture was then heated at 60 °C for 2 min, and immediately supplemented with 100 μl of H2O/CH3CN (90/10) containing 0.1% trifluoroacetic acid to stop the reaction.
For determination of dl-serine, l-serine and d-serine, a 20-μl aliquot of the resultant solution was injected into the HPLC system.25, 30 A reversed-phase ODS column (TSKgel ODS-80Ts (Tosoh, Tokyo, Japan) as column 1) was used for the separation and quantification of total (d- and l-) serine, and the gradient elution of the mobile phase was maintained at a constant flow rate of 0.8 ml min−1. Mobile phase 1a consisted of H2O/CH3CN (90/10) containing 0.1% trifluoroacetic acid, and phases 1b and 1c, of H2O/CH3CN (10/90) containing 0.1% trifluoroacetic acid and CH3CN, respectively. The time program for gradient elution was as follows: 0–25 min 1a:1b:1c=92:8:0; 25–25.1 min linear gradient from 8% 1b to 100% 1b; 25.1–35 min 1a:1b:1c=0:100:0; 35–35.1 min linear gradient from 8% 1b to 100% 1c; 35.1–40 min 1a:1b:1c=0:0:100; and 40.1–60 min 1a:1b:1c=92:8:0. The chiral column (column 2) used for the separation and quantification of d- and l-serine with NBD-F comprised two Sumichiral OA-2500 columns (Sumika Chemical Analysis Service, Osaka, Japan), which were connected in tandem. The mobile phase was 15 mm citric acid in MeOH. The flow rate was isocratically pumped at 1.0 ml min−1. The column temperature of all columns was maintained at 35 °C. Fluorescence detection was performed at 530 nm, with an excitation wavelength at 470 nm.
For determination of glycine, glutamine and glutamate, a reversed-phase ODS column (TSKgel ODS-80Ts, Tosoh) was used.23, 30 The gradient elution of the mobile phase was kept at a constant flow rate of 0.8 ml min−1. The time program for gradient elution was programmed as follows: 0–50.5 min 1a:1b:1c=95:5:0; 50.5–55.5 min 1a:1b:1c=0:100:0; and 55.5–57 min 1a:1b:1c=0:0:100. The column temperature of all columns was maintained at 35°C. Fluorescence detection was performed at 530 nm, with an excitation wavelength at 470 nm.
Statistical analysis
First, Student t- and χ2-tests were used to compare the MDD and comparison groups with respect to dimensions not associated with depression (that is, MMSE score, years of education, body mass index, age, incidence of diabetes, gender, APOE status and reported family history of AD; Table 1). Second, Student t-test was used to compare the two diagnostic groups with respect to all remaining CSF variables. Third, 3-year follow-up data were analyzed using a 2X2 repeated-measures analysis of variance; the factors were time (baseline and follow-up) and MDD status (MDD and controls). All tests were two-tailed, and statistical significance was established at an α of 0.05, unless differently noted. All analyses were conducted using SPSS 22 (SPSS, Chicago, IL, USA).
Results
As reported previously,26 the two groups at baseline did not differ on any relevant clinical or demographic variable, with the exception of the mean HAM-D score, which was significantly higher in the MDD group (Table 1). Of note, the proportion of participants with a reported family history of AD was slightly higher in the comparison group than in the MDD group. The CSF levels of amyloid-β42 in the MDD group at baseline were significantly lower than those of the comparison group, whereas differences in CSF levels of amyloid-β40, and total and phosphorylated tau protein at baseline did not reach statistical significance across conditions (Table 1).26
There were no significant differences in levels of five amino acids (glutamine, glutamate, glycine, l-serine and d-serine) between the MDD (N=28) and comparison groups (N=19) at baseline (Table 2). However, the ratio of glutamine to glutamate in the MDD group was significantly higher than in the comparison group, whereas the ratio of glycine to l-serine and the ratio of d-serine to l-serine did not differ (Table 2). Furthermore, there were no correlations between CSF amino acids and typical AD biomarkers (amyloid-β42, amyloid-β40, and total and phosphorylated tau protein) at baseline in all subjects (data not shown).
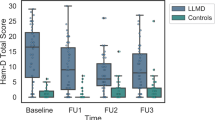
At the 3-year follow-up, an interaction between time and MDD status was observed for the HAM-D scores. Post hoc comparisons confirmed that the severity of depression decreased over time in participants with MDD (P=0.001), whereas no change was detected in controls (P=0.366; Table 3 and Figure 1). When the same repeated-measures analysis of variance was applied to CSF levels of amino acids, no further interactions or main effects were observed when setting α at 0.006 (Sidak correction; eight tests), although a trend was observed for an interaction with the glutamine/glutamate ratio (Table 3 and Figure 1). Post hoc comparisons suggested that there was a decrease in this ratio in MDD subjects (P=0.008), but no appreciable change in controls (P=0.571). Finally, we observed a marginally significant negative correlation (r=−0.328, P=0.051) between the change scores (baseline to follow-up) of the glutamine/glutamate ratio and of HAM-D scores in all subjects, suggesting that as depression severity decreased, so did the ratio.
Three-year follow-up of elderly MDD patients and control comparison. (a) The ratio of glutamine to glutamate in the cerebrospinal fluid (CSF) from major depressive disorder (MDD) patient group (N=19) was significantly decreased at 3-year follow-up. In contrast, the ratio of glutamine to glutamate in the CSF from control comparison group (N=17) was slightly increased at 3-year follow-up. (b) The Hamilton Depression Rating Scale (HAM-D) score in the MDD patient group (N=19) was significantly decreased at 3-year follow-up. In contrast, HAM-D score in the control comparison group (N=17) was slightly increased at 3-year follow-up.
Discussion
In the present study, we found that older individuals with MDD showed an increased CSF glutamine/glutamate ratio compared with controls, although CSF levels of the individual amino acids were not different. To our knowledge, this is the first report in the literature of an increase in the glutamine/glutamate ratio in elderly MDD subjects, suggesting abnormalities in the glutamine–glutamate cycle in the brains of elderly depressed individuals. As the glutamine/glutamate ratio is an index for glutamine–glutamate cycle in the glia–neuron communication,8, 23 it is likely that abnormalities in the glutamine–glutamate cycle in the brain have a role in the pathophysiology of late-life depression.
We previously reported a reduction of amyloid-β42 in the same MDD patients at baseline.26 Importantly, the glutamine/glutamate ratio in elderly MDD patients was no longer significantly different from controls after a 3-year period; the loss of significance coincided with reduction in the severity of depressive symptoms, suggesting that abnormalities in this ratio in depression may be state- and not trait-dependent. However, we found no significant correlations between the ratio or levels of the individual amino acids and AD biomarkers, or severity of depressive symptoms either at the baseline or follow-up. These results are generally consistent with previous findings of CSF studies that also reported a lack of significant correlations between these amino acids and severity of depressive symptoms. Garakani et al.22 reported no significant differences between controls and MDD patients in baseline CSF levels of glutamate (P=0.761) or glutamine (P=0.226). Furthermore, there was a significant positive correlation (r=0.677, P=0.016) between intensity of suicidal ideation and CSF glutamate, and a significant negative correlation (r=−0.558, P=0.038) between baseline HAM-D score and the glutamate/glutamine ratio in adult patients (mean age of 40.4 years) with MDD. It is thus very difficult to reconcile the lack of significant correlations between the CSF ratio and severity of depressive symptoms with evidence that the increased CSF glutamine/glutamate ratio and other glutamatergic abnormalities associated with MDD might be state-dependent. Taken together, these findings highlight the limitations of static indices such as levels of glutamate or glutamine either in CSF or brain to reflect dynamic and brain region-specific alterations in the glutamatergic function. Using in vivo 13C-MRS and [1-13C]glucose in a single voxel spectroscopy study, Abdallah et al.31 found that a measure of the neuronal tricarboxylic acid cycle in the occipital cortex from medication-free MDD patients was 26% lower compared with controls, suggesting mitochondrial dysfunction in depression. However, they reported no significant alterations in the glutamine–glutamate cycle and γ-aminobutyric acid (GABA) synthesis, inconsistent with our CSF findings. Furthermore, this study did not find any correlations with severity of depression.31 One possibility is that the investigators focused only on the occipital cortex, and they did not examine CSF or other brain regions in which glutamatergic abnormalities have been found.
Glutamate is synthesized from glutamine by glutaminase, and metabolized to glutamine by the mainly astrocyte-located GS. In addition, glutamate is metabolized to GABA by glutamic acid decarboxylase (GAD).8 Released glutamate is taken up by surrounding astrocytes via the glutamate transporter, where it is converted to glutamine, transported back to presynaptic neurons and reconverted to glutamate. Thus, the glutamine–glutamate–GABA cycle as part of glia–neuron communication has an important role in excitatory and inhibitory neurotransmission.8 The expression of GS mRNA was reduced in the prefrontal cortex, premotor cortex and amygdala from depressed suicide subjects, but not in suicide completers without depression.32, 33 Furthermore, GS protein was decreased in the anterior cingulate cortex and orbitofrontal cortex from depressed patients.32, 34, 35 A recent study using postmortem brain samples showed that the density of GS-expressing astrocytes was significantly reduced in some cortical areas from MDD patients, suggesting a disturbance in the glutamine–glutamate cycle in MDD.36 Moreover, the immunoreactivity of EAAT2, one of the glutamate transporters in astrocytes, in the orbitofrontal cortex was significantly lower in MDD patients.35 In addition, increased immunoreactivity for GAD65/67 was demonstrated in neurons from several cortical regions, including orbitofrontal and dorsolateral prefrontal cortex, in depressed patients.37 By contrast, the amount of GAD67 in the dorsolateral prefrontal cortex of MDD patients was significantly reduced as compared with matched controls.39 These findings suggest a dysregulation of the glutamate–GABA cycle in depression. Taken together, it is likely that abnormalities in the glutamine–glutamate–GABA cycle in the brain have a role in the pathophysiology of depression.
In this study, we found that the glutamine/glutamate ratio in MDD patients was significantly decreased after 3-year follow-up in concert with improvement in depressive symptoms. Given the role of glutamatergic neurotransmission in the mechanisms of antidepressants,5, 6 it is noteworthy that symptom improvement caused by antidepressants may be mediated by effects on glutamine–glutamate cycle in the brains from depressed patients. In contrast, HAM-D score and the glutamine/glutamate ratio in healthy subjects were slightly increased after 3-year follow-up, although these increases were not statistically significant.
CSF levels of glutamine in individuals with AD were reported to be lower than in controls,40 whereas CSF levels of glutamate were higher.42, 41 Although the glutamine/glutamate ratio was not measured in either study, and depression was not a focus of either investigation, these results do not support a link between the abnormalities in the glutamine–glutamate cycle in depression with those in AD. Increased CSF levels of GS in individuals with AD compared with controls also have been reported.43, 44 In contrast, a recent study using a quantitative ELISA system showed that CSF levels of total GS in AD patients were not different from those observed in healthy controls,38 suggesting that total CSF levels of GS may not be a suitable biomarker for AD, or that further work needs to be done with this biomarker to clarify whether GS abnormalities exist in AD that are associated with the apparent abnormalities in mood disorders.
The NMDA-R antagonist ketamine is known to produce a rapid and sustained antidepressant effect in treatment-resistant patients with MDD.11, 12 A recent MRS study demonstrated that a single infusion of ketamine (0.5 mg kg−1) caused an increase of Glx and GABA in the medial prefrontal cortex of MDD patients in ~26 min,45 indicating that ketamine has a rapid effect on the glutamine–glutamate–GABA cycle in the prefrontal cortex in depressed patients. In contrast, earlier studies showed that a single infusion of ketamine (0.5 mg kg−1) did not produce changes in glutamate and glutamine concentrations in occipital cortex in MDD patients,46 or in the anterior cingulate/medial prefrontal cortex in healthy subjects,47 suggesting that changes in occipital amino-acid levels are not correlated with ketamine’s antidepressant action. Thus, it would be of great interest to measure CSF amino acids in MDD patients at baseline and after a single ketamine infusion.
Finally, there are some limitations to this study that should be noted. The main limitation was small sample size, and similar, future studies in geriatric depression would likely benefit from larger sample sizes. Another limitation was the inability to measure CSF GABA in the subjects of this study. We could not measure CSF levels of GABA because these were below the lower limit of detection of our HPLC system. Given the key role of glutamine–glutamate–GABA cycle in glia–neuron communication,5, 8 it is of great interest to measure CSF GABA levels using a high-sensitive analytical system (for example, liquid chromatography coupled with tandem mass spectrometry).
In conclusion, we found that the CSF ratio of glutamine/glutamate levels in elderly patients with MDD was significantly higher than that of age-matched healthy controls, and that the increased ratio in patients was significantly decreased after 3-year follow-up by medication in conjunction with decreased depression symptoms over this time period. These findings suggest that abnormalities in the glutamine–glutamate cycle in the glia–neuron communication have a role in the pathophysiology of elderly depression. Further studies measuring CSF levels of amino acids (for example, glutamine, glutamate and GABA) using larger cohorts, particularly cohorts of antidepressant-naive patients, will be of great interest.
References
Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML . High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry 2010; 67: 489–496.
Diniz BS, Butters MA, Albert SM, Dew MA, Reynoilds CF . Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202: 329–335.
Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D . Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006; 63: 530–538.
Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA . Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012; 69: 493–498.
Hashimoto K . Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 2009; 61: 105–123.
Hashimoto K, Malchow B, Falkai P, Schmitt A . Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 2013; 263: 367–377.
Hertz L . The glutamate-glutamine (GABA) cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front Endocrinol (Lausanne) 2013; 4: 59.
Hashimoto K . Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin Ther Targets 2014; 18: 1049–1063.
Sanacora G, Zarate CA, Krystal JH, Manji HK . Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008; 7: 426–437.
Zarate C Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G . Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry 2010; 18: 293–303.
Krystal JH, Sanacora G, Duman RS . Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 2013; 73: 1133–1141.
Sanacora G, Schatzberg AF . Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 2015; 40: 259–267.
Hashimoto K, Sawa A, Iyo M . Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 2007; 62: 1310–1316.
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004; 61: 705–713.
Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F . Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 2000; 47: 305–313.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC . Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64: 193–200.
Godlewska BR, Near J, Cowen PJ . Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 2015; 232: 501–507.
Hermens DF, Naismith SL, Chitty KM, Lee RS, Tickell A, Duffy SL et al. Cluster analysis reveals abnormal hippocampal neurometabolic profiles in young people with mood disorders. Eur Neuropsychopharmacol 2015; 25: 836–845.
Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J . Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol 2015; 25: 1109–1117.
Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW . Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry 2000; 47: 586–593.
Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM . Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry 2007; 61: 162–166.
Garakani A, Martinez JM, Yehuda R, Gorman JM . Cerebrospinal fluid levels of glutamate and corticotropin releasing hormone in major depression before and after treatment. J Affect Disord 2013; 146: 262–265.
Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindstrom LH, Iyo M . Elevated glutamine/glutamate ratio in cerebrospinal fluid of first episode and drug naïve schizophrenic patients. BMC Psychiatry 2005; 5: 6.
Hashimoto K . Abnormalities of the glutamine-glutamate-GABA cycle in the schizophrenia brain. Schizophr Res 2014; 156: 281–282.
Pålsson E, Jakobsson J, Södersten K, Fujita Y, Sellgren C, Ekman CJ et al. Markers of glutamate signaling in cerebrospinal fluid and serum from patients with bipolar disorder and healthy controls. Eur Neuropsychopharmacol 2015; 25: 133–140.
Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry 2012; 169: 523–530.
Buschke H . Retrieval in human learning. Trans N Y Acad Sci 1974; 36: 721–729.
Army Individual Test Battery Manual of Directions and Scoring. Adjutant General’s Office, War Department: Washington, DC, USA, 1994.
Goodglass H, Kaplan E . Assessment of Aphasia and Related Disorders. Lea & Febiger: Philadelphia, PA, USA, 1972.
Fukushima T, Kawai J, Imai K, Toyo’oka T . Simultaneous determination of D- and L-serine in rat brain microdialysis sample using a column-switching HPLC with fluorimetric detection. Biomed Chromatogr 2004; 18: 813–819.
Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL et al. Glutamate metabolism in major depressive disorder. Am J Psychiatry 2014; 171: 1320–1327.
Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA 2005; 102: 15653–15658.
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One 2009; 4: e6585.
Beasley CL, Pennington K, Behan A, Wait R, Dunn MJ, Cotter D . Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: evidence for disease-associated changes. Proteomics 2006; 6: 3414–3425.
Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA . Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord 2010; 127: 230–240.
Bernstein HG, Meyer-Lotz G, Dobrowolny H, Bannier J, Steiner J, Walter M et al. Reduced density of glutamine synthetase immunoreactive astrocytes in different cortical areas in major depression but not in bipolar I disorder. Front Cell Neurosci 2015; 9: 273.
Bielau H, Steiner J, Mawrin C, Trübner K, Brisch R, Meyer-Lotz G et al. Dysregulation of GABAergic neurotransmission in mood disorders: a postmortem study. Ann N Y Acad Sci 2007; 1096: 157–169.
Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G . Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 2010; 13: 411–420.
Smith CC, Bowen DM, Francis PT, Snowden JS, Neary D . Putative amino acid transmitters in lumbar cerebrospinal fluid of patients with histologically verified Alzheimer’s dementia. J Neurol Neurosurg Psychiatry 1985; 48: 469–471.
Csernansky JG, Bardgett ME, Sheline YI, Morris JC, Olney JW . CSF excitatory amino acids and severity of illness in Alzheimer's disease. Neurology 1996; 46: 1715–1720.
Pomara N, Singh R, Deptula D, Chou JCY, Schwartz MB, LeWitt PA . Glutamate and other CSF amino acids in Alzheimer’s disease. Am J Psychiatry 1992; 149: 251–254.
Gunnersen D, Haley B . Detection of glutamine synthetase in the cerebrospinal fluid of Alzheimer diseased patients: a potential diagnostic biochemical marker. Proc Natl Acad Sci USA 1992; 89: 11949–11953.
Tumani H, Shen G, Peter JB, Brück W . Glutamine synthetase in cerebrospinal fluid, serum, and brain: a diagnostic marker for Alzheimer disease? Arch Neurol 1999; 56: 1241–1245.
Timmer NM, Herbert MK, Claassen JA, Kuiperij HB, Verbeek MM . Total glutamine synthetase levels in cerebrospinal fluid of Alzheimer’s disease patients are unchanged. Neurobiol Aging 2015; 36: 1271–1273.
Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry 2015; 10.1038/mp.2015.83; e-pub ahead of print 18 August 2015.
Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res 2011; 191: 122–127.
Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ . Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol 2012; 26: 733–737.
Acknowledgements
We acknowledge Ms Yuko Fujita (Chiba University, Japan) for her technical assistance in the measurement of amino acids. This research was supported by grants from Comprehensive Research on Disability, Japan Agency for Medical Research and Development (AMED) (to KH, H26-seishin-ippan-015); the Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to KH, 24116006); the Grant-in-Aid for Challenging Exploratory Research, JSPS (to KH, 15K15423); the Torsten Söderberg Foundation at the Royal Swedish Academy of Sciences; the Knut and Alice Wallenberg Foundation; the Swedish Research Council; and the NIMH (to NP, R01 MH-080405). We are also thankful to the patients and controls participating in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hashimoto, K., Bruno, D., Nierenberg, J. et al. Abnormality in glutamine–glutamate cycle in the cerebrospinal fluid of cognitively intact elderly individuals with major depressive disorder: a 3-year follow-up study. Transl Psychiatry 6, e744 (2016). https://doi.org/10.1038/tp.2016.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.8
This article is cited by
-
The role of gut microbiota in the pathogenesis and treatment of postpartum depression
Annals of General Psychiatry (2023)
-
Luvadaxistat: A Novel Potent and Selective d-Amino Acid Oxidase Inhibitor Improves Cognitive and Social Deficits in Rodent Models for Schizophrenia
Neurochemical Research (2023)
-
Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications
Molecular Psychiatry (2022)
-
Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor
Molecular Psychiatry (2022)
-
Metabolomics changes in brain-gut axis after unpredictable chronic mild stress
Psychopharmacology (2022)