Abstract
Studying host parasite interactions and their implications for evolution and ecology recently received increasing attention, particularly with regard to host physiology and immunity. Here we assess variation of urinary neopterin (uNEO), a marker of cellular immune activation and iummunosenescence, in response to age and anthelmintic treatment in semi-free ranging Barbary macaques (Macaca sylvanus). Urinary NEO levels were measured via enzyme-immunoassay from 179 urine samples of 43 individuals between 5–29 years of age. Efficiency of treatment was assessed by Mc Master flotation on repeated faecal samples, including 18 untreated individuals as control group. We used linear mixed models with age and parasite status as main effects, controlling for sex and physical condition, assessed through urinary C-Peptide-levels, with social group and ID as random factors. Urinary NEO levels significantly increased with age, suggesting that changes in aging Barbary macaque immune responses are consistent with immunosenescence described in human and nonhuman primates and can be detected via uNEO measurements. Anthelmintic treatment, however, had no influence on uNEO levels, potentially due to quick reinfections or attenuated immune responses in repeated infections. We conclude that uNEO is a potential non-invasive marker for immune function and particularly immunosenescence in wildlife.
Similar content being viewed by others
Introduction
Infectious diseases have crucial impacts on the ecology and evolution of species, influencing numerous aspects of host life history, survival and fitness1,2 and are considered a major cost of group living3. Infections have been studied intensely in humans4,5 and model species6,7, and recently investigations of wildlife diseases and immune response have received increasing attention8. Recent advances in the field of studying senescence in wildlife9 demonstrate that senescence occurs in wild animals and consistently changes their physiology and immune response10,11. To understand the importance of age related changes and host-pathogen interactions for evolution, studying diseases and immune responses in natural systems has become crucially important. Wildlife health monitoring has focused on different aspects of an animal’s physiology that can be measured non-invasively, e.g. glucocorticoid excretion as a measure of physiological stress response12,13, testosterone excretion due to its supposed, though debated, immunosuppressive effect14,15, monitoring physical condition using visual criteria16,17, wound healing18, and measurements of C-Peptide of insulin19,20 and thyroid hormones21,22 as markers for energetic status. Studies of age related changes currently rely mainly on invasive sampling10,11, however, capturing and handling animals can be difficult or impossible and raise particular ethical concerns for endangered species or species sensitive to disturbance. Problems associated with invasive sampling make comparative work on health status challenging, so the development of non-invasively obtained measures of health- and age-related parameters are critical to this area of inquiry.
Information about immune responses of individuals with regard to certain pathogens and aging are necessary to understand the impact of infection on host fitness, as immune responses themselves are energetically costly or lead to energy allocation away from reproduction23. Using blood samples is highly efficient when studying immune responses on the molecular level and have generated major insights into host-parasite interactions24 and the effects of intrinsic and extrinsic factors on immune responses and reactivity10,25. One current challenge in the fields of wildlife senescence and eco-immunology is the validation and establishment of suitable markers to monitor immune responses non-invasively (e.g. using faecal and urine samples) in order to better understand host parasite interactions and their implications for fitness in a wider range of systems.
Recently, advances have been made validating assays to measure markers of immune response in faecal and urine samples from nonhuman primates26. One particularly promising marker is neopterin (NEO)26,27, which is released from macrophages, monocytes and dendritic cells in response to interferon gamma (INFγ) stimulation28 and induces T-helper 1 cell activation in the immune response against intracellular pathogens such as viruses and bacteria28,29. Being also elevated in a variety of inflammatory30 and non-infectious diseases28,31 and even under acute stress32, NEO may serve as a non-disease-specific marker of inflammation and Th1 mediated immune responses and is widely used as a diagnostic marker for disease severity and prognosis in humans in a variety of diseases28. NEO is cleared unchanged via the kidneys33 and serum and urinary NEO levels are strongly correlated in both human34 and nonhuman primates26,35. Supporting limited findings of an earlier study35, uNEO levels increased more than 10-fold following infection with Simian immunodeficiency virus (SIV)26, indicating the usefulness of uNEO level changes as a non-invasive marker of viral infection in nonhuman primates.
Apart from being a marker of acute or chronic disease associated with a Th1 immune response28,29, NEO is a marker intensely studied with respect to age-related changes in the immune system. There are consistent changes in both, the innate and adaptive immune system, occurring with older age: decreased cellular immune response36 and efficiency of vaccinations37,38, changes in T-cell phenotypes with an increase in differentiated and simultaneous decrease in naïve T-cells39,40, alterations in the innate immune system, especially changed monocyte phenotypes41 as well as reduced natural killer cell function40,42, and chronic low levels of inflammation40,43. Elevated NEO levels in aged individuals have not only been reported in numerous studies44,45, but also correlate with several hallmark characteristics of the aged immune system in humans, amongst others altered T-cell phenotypes and changes in monocytes41,45, indicating NEO as a marker of age related immune function changes.
Given the growing importance of understanding healthy aging and the processes leading to frailty and morbidity with older age in aging human societies46, nonhuman primates have become an increasingly important model system to study immunosenescence47,48, a term that summarizes the changes in the immune system of aging individuals49. To date, most studies on immunosenescence in nonhuman primates report levels of IFNγ rather than NEO, which in most studies are elevated in older individuals50,51. Since IFNγ and NEO are closely linked functionally28, similar patterns are expected for NEO in aging nonhuman primates. This is supported by a recent study that reported a significant positive correlation between serum NEO levels and age in healthy macaques26.
We aimed at advancing the biological validation of uNEO in nonhuman primates with regard to aging by using a cross-sectional design of young adult to aged free ranging Barbary macaques (Macaca sylvanus). Additionally, we aimed at assessing whether uNEO levels decrease in response to gastrointestinal (GI-) nematode infection, a group of pathogens that have been widely studied in nonhuman primates12,52 and other taxa53,54. We capitalized on routine six-monthly anthelmintic treatment and assessed the impact of parasite clearance on uNEO levels in a cross-sectional design. Since both arms of the immune system, Th1 and Th2 are mutually inhibitory55, high levels of NEO not only represent an efficient Th1, but also an inhibited Th2 response. As immune responses against GI-nematodes generally share a strong antibody mediated and anti-inflammatory Th2-type response56, uNEO can potentially function as not only a direct marker of intracellular, but also an indirect marker of parasite infections. A similar relationship between GI parasite infection and Th1 responses was demonstrated in wild African buffalos (Syncerus caffer), where individuals with lower Th1 activity were less vulnerable to GI-nematode infections24 and Th1 responses increased as a result of experimental parasite clearance. Since direct energetic costs of immune responses, including parasite defences, have been shown in several taxa57,58, and clinical studies on humans have linked NEO levels to markers of energy status59,60, we integrated a non-invasive measure of energy balance, urinary C-Peptides (uCP)20,61, into our analyses to control for possible confounding effects of physical condition on uNEO values. We investigated the relationship between aging, GI parasite infection and uNEO levels in semi-free ranging Barbary macaques at Affenberg Salem in a population with a high proportion of aged individuals older than 20 years (19% of the study population). We specifically predicted increasing uNEO levels with increasing individual age in adulthood, representing changes in the immune system consistent with immunosenescence. Individuals older than 20 years were expected to have the highest uNEO levels. Additionally, we used routine anthelmintic treatment of the population to investigate the impact of GI parasite clearance on uNEO. Based on the findings of Ezenwa et al.24,54, we predicted uNEO levels to be increased after treatment due to lack of immunomodulation by parasites.
Materials and Methods
Study site and urine sample collection
We studied two out of three freely interacting social groups of Barbary macaques in a 20 ha forested enclosure at Affenberg Salem in Germany. Macaques were provided with fruit, vegetables, and grains daily in the morning and had ad libitum access to monkey chow and water62. The two study groups had roughly similar age-sex compositions (group C: 20 adult females, 16 adult males, 11 immature females and 12 immature males; group H: 23 adult females, 18 adult males, 3 immature females, 8 immature males). All individuals were identifiable by physical features such as birth marks, scars, stiff fingers or stature. Urine samples from all adult individuals were collected repeatedly from individually recognized individuals six weeks prior to anthelmintic treatment to allow for collection of baseline pre-treatment data for the study population. Sampling was continued until four weeks post-treatment (group C: 2nd of June through 26th of August 2014, group H: 6th of June through the 9th of September 2015). The four weeks window was chosen to allow for collection of several samples per individual while being able to assess the effect of reduced parasite burden. Since prepatant phases of strongyle nematodes present in the population are approximately two to three weeks63 and most individuals started shedding eggs six weeks after treatment or later (unpublished data), we are confident this was actually the case. Only samples of 43 adult individuals (7 of group C, 36 of group H) with at least 1 sample pre- and post-treatment were analysed (179 samples, 4.2 ± 1.3/individual, pre-treatment: 93 samples, 2.2 ± 0.8/individual, post-treatment: 86 samples, 2.0 ± 0.9/individual). The 22 sampled females were 5–29 years of age and the 21 males 6–27 years of age (Table 1). Samples were taken non-invasively using one of two methods: Urine was caught on clean plastic sheets when individuals were urinating from elevated positions (higher than 2 m) and subsequently transferred into 2 ml polypropylene cups with disposable Pasteur pipettes. We aimed at collecting samples from every individual, but had difficulties to obtain samples in 2014 as Barbary macaques are largely terrestrial and samples could not be collected when individuals urinated close to the ground. In 2015 we increased our sampling effort in order to obtain samples from more individuals. In addition to collecting urine by catching it on plastic sheets we collected urine from the ground, rocks or leaves using swabs (Salivette® Cortisol, Sarstedt, Nürmbrecht, Germany) when individuals urinated close to or on the ground. The latter method has recently been validated for NEO measurements in macaque and human urine64 and allowed for a much higher sampling success. Samples contaminated with faeces were discarded as faecal contamination alters levels of uCP in macaque urine samples65. Following collection, both salivettes and polypropylene cups were stored on ice in a thermos flask until transferring them to −20 °C for long-term storage within 12 h of collection27. Urine was recovered from the salivettes by centrifugation using a manually operated centrifuge64 and was transferred to 2 ml polypropylene cups with disposable Pasteur pipettes prior to freezing. All urine samples were transported to the Endocrinology Laboratory of the German Primate Centre (Göttingen) on dry ice and stored frozen at −20 °C until analysis.
Anthelmintic treatment, parasite analyses and treatment efficacy
Anthelmintic treatment was performed on the 5th of August 2014 and 17th of August 2015 as a veterinary routine measure. Individuals were fed food items containing ivermectin, a broad spectrum anthelmintic compound belonging to the macrocyclic lactone drug class, at approximately 0.4 mg/kg bodyweight, by park staff in close collaboration with the veterinarian responsible for the population. In 2015, half of the individuals of group H remained untreated as a control group (n = 18), which was matched for sex, age, immigration status (males) and matriline (females) to the treatment group (nine females, 5–29 years, nine males, 6–27 years of age).
Since we aimed at investigating the impact of age and parasite status on uNEO levels, confirming the efficacy of anthelmintic treatment was one prerequisite for the analyses. To do so we collected faecal samples for every individual approximately once per week for parasite analyses (pre-treatment: n = 294, 6.7 ± 0.9/individual, post-treatment n = 292, 3.8 ± 1.4/individual). Samples were collected as soon as possible (without disturbing the animal) after defecation noting animal ID, date, time, and observer, placed in 20 ml plastic tubes, stored on ice until being fixed with 10% formalin within 12 hours and later transported to the Institute for Parasitology of the University of Veterinary Medicine Hannover, Germany. Presence of nematode eggs was assessed using McMaster flotation, a quantitative parasitological method, after wash-out of formalin from the samples by transferring ca. 6 g to a 15 ml centrifuge tube, spinning at 2000 rpm (930 g) for 10 min, washing once with water, spinning at 2000 rpm (930 g) for 10 min and discarding the supernatant66. For McMaster flotation, 4 g faecal matter was weighed, homogenized in saturated NaCl as flotation solution, poured through a tea strainer (mesh size 1 mm) to remove faecal particles and the sample was filled up to 60 ml with saturated NaCl. For samples with less faecal matter available, 15 ml flotation solution were used per gram and the weight of faeces used was recorded. Faecal egg counts were performed by using McMaster chambers and scanning 4 counting fields with 100- and 400-fold magnification (detection sensitivity 25 eggs/gram faeces), recording presence of parasite egg morphotypes.
Coproscopical parasite analysis revealed three nematode morphotypes, for which effectiveness of treatment differed: for strongyle nematodes, which had a pre-treatment prevalence of ~98% (n = 42 individuals), all treated individuals stopped shedding eggs within two days of treatment and stayed coproscopically negative for at least three weeks. One individual excreted eggs again within three weeks and three individuals within four weeks. All other treated individuals remained coproscopically negative for at least four weeks after treatment. As strongyle eggs are difficult to differentiate by morphological characteristics, we summarized several potential genera under this morphotype, e.g. Oesophagostomum spp. and hookworms (Ancylostoma spp. and Nercator spp.). Preliminary results from larval cultures suggest that the majority of strongyles represent Oesophagostomum spp., whereas only few samples contained other larval morphotypes (unpublished data). Given the short prepatent periods of the possible parasite species63, this result indicates parasite clearance with rapid reinfection in few individuals. For the two other morphotypes, Capillaria spp. and Trichuris spp., prevalences were lower (Capillaria spp.: 37.2%, n = 16 individuals; Trichuris spp.: 6.9%, n = 3 individuals), but anthelmintic treatment was not efficient for all treated individuals as indicated by continued egg shedding (two study individuals and seven additional treated individuals not included in the study for lack of urine samples).
uNEO and uCP measurements
We assessed uNEO concentrations measuring duplicates for each sample using a commercial Neopterin ELISA Kit (Art. No. RE59321, IBL International GmbH, Hamburg, Germany) previously validated for the use in macaques26,27. Prior to assay, urine samples were diluted 1:25 to 1:250 with assay buffer and 20 μl of the diluted urine was subsequently assayed using the manufacturer provided protocol. In this study, uNEO concentrations are provided as ng/ml by transforming the respective nmol/l values according to the manufacturer’s protocol. Sensitivity of the assay was 0.34 ng/ml. Intraassay coefficients of variation (CV), determined by repeated measurement of high and low value quality controls in each assay, were 5.4% (high; n = 18) and 8.1% (low; n = 18), respectively, while interassay CVs were 6.1% (high; n = 5) and 10.5% (low; n = 5).
In addition to uNEO levels, we measured uCP concentrations as a control factor to account for individual differences in physical condition and energy balance19,20 as these might influence the ability of individuals to mount effective immune responses58,67 and influence uNEO levels57,59,60. We analysed uCP measuring duplicates of each sample using a commercial C-Peptide ELISA Kit (Art. No. RE 53011, IBL International GmbH, Hamburg, Germany) previously validated for the use in macaques20,65. Prior to assay, urine samples were diluted between 1:2 and 1:20 with IBL sample diluent (Art. No. RE 53017) and 100 μl of the diluted urine was then assayed according to the manufacturer’s instructions. Assay sensitivity was 0.064 ng/ml. Intraassay CVs calculated from the measurement of high- and low-value quality controls (pooled human urine) were 5.2% (high; n = 18) and 8.8% (low; n = 18), respectively, while values for interassay CVs were 7.0% (high; n = 5) and 14.2% (low; n = 5).
Due to the expected sex differences in muscle mass and consequently creatinine excretion68, we did not use creatinine but specific gravity (SG) to adjust for differences in urine volume and concentration68. We measured SG of each sample using a digital hand-held refractometer (PAL-10S; Atago Inc., Bellevue, USA) and calculated uNEO levels and uCP levels corrected for SG by using the following formula69: 
Statistical analysis
We assessed the effect of age and anthelmintic treatment on uNEO levels using linear mixed models in R (version 3.570) with the package lmerTest, setting alpha levels to 0.05. After exclusion of two extremely low uNEO values that repeatedly led to extremely high residuals and prohibited log-transformation to achieve normality, both uNEO as response and uCP as predictor were log-transformed to achieve normal distribution. Rather than z-transforming age, age was rescaled by subtracting the mean age (14 years) value from individuals’ values to centre the model around the mean age rather than the not meaningful value of age = 0 for the y-intercept. This was done to keep the original data structure and allow interpretation of the effect size.
Since Trichuris always occurred in individuals positive also for Capillaria both morphotypes were summarized under the factor “Cap” for statistical analysis. Based on the differences in anthelmintic treatment efficacy and the different outcomes for Cap negative (GI parasite free) vs. Cap positive (GI parasites still present), we modelled the predictors representing parasite treatment using a three-way interaction between experimental group (control vs. treatment), phase (pre- vs. post-treatment) and Cap (positive vs. negative) as main factor in the original model (Model 1), expecting smaller or no changes in uNEO levels in Cap positive vs. Cap negative treated individuals. The model contained the three-way interaction and age as main factors. As NEO has been shown to increase in elderly humans only rather than linearly across adulthood, we tested using a model with age as a quadratic term to account for the nonlinearity, however, the quadratic term was neither significant (estimate age2 = −0.001, t-value = −0.498, p = 0.621) nor improving the model fit (model comparison using a likelihood ratio test with the R function anova, setting the argument test to “Chisq”, Chisq = 0.390, p = 0.533), so we chose a linear fit for age. We included sex as a control variable, because rhesus macaque females showed higher levels than males in laboratory studies, though this effect was not significant26. The two-way interactions of Cap, experimental group and phase were included, albeit being not interpretable given the three-way interaction, as required for model validity. The single terms Cap, experimental group, phase as well as sex and uCP were included as control factors, social group and animal ID as random factors.
Since the three-way interaction was not significant we tested if its inclusion significantly improved the model fit as a measure of model selection. Model 1 was not significantly better fitted to explain uNEO level variability than the identical model including only the two-way interaction between experimental group and phase and the single terms experimental group, phase and Cap (Chisq = 5.369, p = 0.147). Consequently, we used the less complex model for interpretation of the results (Model 2), but report results of Model 1 for reasons of transparency.
For both models, various model diagnostics were employed to confirm model validity (visual inspection of distribution of residuals, qqplots, residuals plotted against fitted values, assessing model stability using the function “glmm stability” written by Roger Mundry (MPI Evolutionary Anthropology, Leipzig), assessing leverage and dfbetas for single samples and levels of the random factor ID using the package “influence. ME” and variance inflation factors using the package “car”), none of which suggested violation of model assumptions for either model. To assess the significance of the full models we compared them to reduced models excluding the age-term and the interaction-term between experimental group and phase while retaining all other predictors using a likelihood ratio test with the R function anova, setting the argument test to “Chisq”.
Ethical statement
This work followed the Animal Behaviour Society’s guidelines for the treatment of animals in behavioural research and teaching, and adhered to standards as defined by the European Union Council Directive 2010/63/EU on the protection of animals used for scientific purposes. Anthelmintic treatment was performed as part of the routine procedures of Affenberg Salem as defined by the European Union Council Directive 1999/22/EC and authorized by the Veterinary Office of the district office of county Lake Constance. The study was approved by the Animal Welfare Body of the German Primate Center (No. E9-16).
Results
Influence of age on uNEO in semi-free ranging Barbary macaques
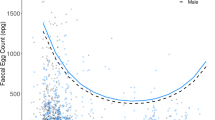
This study was the first to measure uNEO in Barbary macaques. Urinary NEO levels varied between 30.7 and 572.7 ng/ml corr. SG, mean ± sd = 160.45 ± 90.50 ng/ml corr. SG (Table 2) and did not differ between the sexes in either of our models (Model 1: estimate ± se = 0.11 ± 0.10, t-value = 1.08, p = 0.29, Model 2: estimate ± se = 0.08 ± 0.10, t-value = 0.83, p = 0.41, see Table 3, Table 4). In accordance with our predictions, age was a significant predictor of uNEO levels, with older individuals showing higher uNEO levels (Model 2: estimate ± se = 0.02 ± 0.01, t-value = 2.45, p = 0.019). The size of the effect was rather small, with individuals increasing ~4% of the mean log(uNEO) in ten years (Fig. 1). However, the age effect was highly stable as indicated by the narrow confidence intervals (2.5%: 0.004, 97.5%: 0.034, see Table 4), and effect sizes are difficult to interpret due to the log-transformation of uNEO levels. Comparing the mean untransformed values between young adult (5–7 years) and aged individuals (>20 years), the difference translates into a 60% increase in uNEO levels from young to aged individuals (Table 2).
Regression line was fitted according to the estimates of Model 2 (n = 179 samples), broken lines depict 95% confidence intervals. Log-transformed uNEO-values have been plotted against the original age values to allow easier interpretation instead of age scaled to mean age used in the model (which changes the y-intercept, but neither estimates nor interpretation of the model).
Influence of anthelmintic treatment on uNEO levels
Including the three-way interaction of Model 1 changed the estimates, but not the directions of the effects (Table 3, Table 4) and did not improve the model fit, thus only Model 2 is discussed here. For Model 2, the full model was significantly better at explaining uNEO variance than a reduced model excluding age and the interaction-term between experimental group and phase (Chisq = 12.18, p = 0.032). Neither of the control factors (sex, Cap, uCP levels, single terms of experimental group and phase) had a significant effect on uNEO levels (Table 4). Contrary to predictions, we found no effect of treatment (interaction between experimental group and phase) on uNEO levels (Model 2: estimate ± se = 0.11 ± 0.13, t-value = 0.81, p = 0.42, Table 4), indicating that clearance of strongyle nematodes did not lead to significant changes in uNEO levels (Fig. 2).
Depicted are least square means ± se and 95% confidence intervals of least square means for Model 2 for control and treatment group pre and post anthelmintic treatment (n = 179 samples: n control pre = 40 samples, n control post = 41, n treatment pre = 53, n treatment post = 45), visualizing the modelled interaction between experimental group and phase.
Discussion
In the present study, we aimed at investigating the effect of aging on uNEO in semi-free ranging Barbary macaques. Capitalizing on routine anthelmintic treatment, we assessed the potential impact of GI-nematode infection and age on uNEO levels. Anthelmintic treatment was successful for strongyle nematodes, whereas Capillaria and Trichuris infections prevailed in some individuals, which was taken into account when analysing the effect of treatment on uNEO levels. We ran linear mixed models to assess the impact of age and anthelmintic treatment on uNEO levels and found that only age showed a significant positive effect on uNEO levels, with aged individuals showing significantly higher levels than younger ones.
In both humans and the macaques in our study, NEO levels increase with age within adults, placing our results alongside findings from numerous studies investigating the link between NEO levels and aging44,59,71,72,73,74. The effect of aging on NEO in nonhuman primates has not been intensively studied, but the overall processes leading to immune system alterations with age seem to be similar to those in humans47,48. IFNγ, which is functionally closely linked to NEO and induces NEO release28,29, rather than NEO itself, is usually measured in laboratory nonhuman primate studies. Despite some inconsistencies the majority of those studies report an increase of IFNγ with older age50,51, consistent with our finding of elevated uNEO levels in aged individuals. Urinary NEO levels in our study were not linked to variation in energy balance because levels of uCP did not explain variation in uNEO levels over and above the effect of age. This suggests that increased uNEO levels in older individuals are not mediated by potential differences in nutritional status59,60 confounded with age.
Age effect size in this study was small, with a change of 4% in ten years in log-transformed uNEO levels. Elevated NEO levels are usually reported in adolescents and elderly, but not throughout adult life75,76, so we tested for a non-linear effect of aging. This was not significant, making linear modelling the better approach. Apart from the possibility that there are interspecific differences in the onset of increasing NEO levels with age between humans and macaques, this result may be driven by the lack of subadult individuals in our sample. Future studies will help to shed a light on the impact of age on uNEO in nonhuman primates. However, setting our results in context with previous results from humans including healthy adults, our results are comparable with respect to the small effect in adults59,72,73. Comparing the mean values of the youngest (5–7 years) and oldest (>20 years) individuals in our study group, log-transformed uNEO levels increased by 10%, which is comparable to a human study that reported a 20% increase in participants from ages 20 to 69 years73.
One of the major challenges in studying immunosenescence is to disentangle changes due to older age from changes due to underlying age-related disease45,74. One alternative or additional explanation for small effect size of age in our study could be the presence of many “healthy agers” more resilient to immunosenescence in our study population, since individuals more susceptible to frailty and disease are less likely to survive to old age. For this reason, very old individuals were excluded in previous studies of immunosenescence in nonhuman primates50. Human cross-sectional studies have demonstrated that, although increases in NEO levels are a highly consistent pattern in healthy aged individuals, this development is aggravated when combined with age related disease or overall worse health: In human gerontological studies, high NEO or IFNγ have been repeatedly linked to increased morbidity (e.g. the occurrence of rheumatoid arthritis77), frailty71 and cognitive decline78. In one longitudinal study on elderly people, the probability of surviving until the end of the study was linked to NEO levels, with higher NEO levels relating to lower survival probability79. Integrating NEO measurements into long-term projects and investigating the link between NEO levels, age and survival retrospectively will shed further light on whether this finding holds true in nonhuman primates.
Considering our findings, we conclude that uNEO has considerable potential as a non-invasive marker of immunosenescence in nonhuman primates. In general, immunosenescence mainly causes changes in T-cell subpopulations36,39, changes in monocyte or natural killer cell populations41,42, chronic, low level inflammation43 and overall poorer immune system performance in response to challenges, e.g. vaccination37,80, none of which can be investigated non-invasively to date. However, these immune system changes correlate with increased NEO levels41. Additionally, adverse health factors, such as HIV infection40, inflammatory processes and prolonged physiological stress36,43 have been shown to induce changes in the immune system that mirror immunosenescence. These are assumed to accelerate and intensify age related changes in the immune system while being linked to elevated NEO levels40,41. Thus, uNEO could serve as an immunosenescence marker, as well as a marker for overall health in wildlife, with chronically elevated uNEO levels indicative of worse health and lower expected survival time in aged individuals. Despite intensive efforts to define consistent changes throughout life history in humans74,75,76, defining markers and threshold values for healthy aging remains challenging. NEO offers a valuable addition to studies of health and aging in wildlife, particularly if combined with other health-linked parameters, such as glucocorticoid hormone levels, visual frailty, wound healing patterns, or energy status, when addressing question of eco-immunology, health and fitness under natural conditions.
The picture concerning the impact of parasites on uNEO levels is less clear, as we found no changes of uNEO levels in response to anthelmintic treatment. Based on the general pattern of immune responses against GI parasites and previous findings in buffaloes24,54, where parasite clearance increased Th1-activity in treated individuals24,54, we predicted an increase in uNEO levels in response to anthelmintic treatment. Since NEO is connected to Th1 responses28,81, inflammation28,71, acute stress32,71 and severity of various infectious and non-infectious diseases in humans28, the lack of evidence for an impact of parasite removal on uNEO levels in this study is surprising. However, several factors could lead to the effects of treatment being masked or attenuated in our study: First, the anthelmintic compound used for treatment, ivermectin, has been reported to improve immune function in studies on various species, e.g. humans, sheep, pigs, rabbits and rats82,83,84,85. However most studies used long-term treatment rather than single doses82, higher than therapeutic dosages83, ivermectin treatment in absence of parasites, or experimental infections with a single parasite species85,86 and reported only transient effects of ivermectin83. Therefore, we conclude that the impact of the ivermectin treatment on uNEO levels in our study is probably negligible.
Second, different parasite species elicit distinct and highly specific immune responses. Both hookworms and Oesophagostomum spp. have been demonstrated to elicit mixed Th1/Th2 type responses87 and Oesophagostomum spp. tend to cause chronic rather than transient infections88, potentially due to a Th1-directed shift and lack of protective immunity87. Likewise, Trichuris infections are usually transient88, but can become chronic if animals fail to mount efficient Th2-responses89. These shifts towards a more Th1 prone response are not only based on host genetics24,89, but can also be induced by the parasites themselves in an arms-race with their host. Evidence is mounting that GI parasites actively manipulate the host immune system via excretory substances90, e.g. both human hookworms and murine Trichuris produce substances that mimic IFNγ or induce its release91,92. Consequently, both immunomodulation by the parasites and parasite clearance can cause heightened Th1 responses24,92, leading to unchanged uNEO levels after treatment.
Third, several individuals in our study became GI parasite positive again quickly after treatment, as indicated by shedding of parasite eggs within four weeks after treatment. This could explain the difference between our lack of finding and the results of studies on ungulates where individuals treated with a compound bolus were parasite free for up to 24 months24,54. We can expect tissue damage due to either wandering hookworm larvae91,93 or encystation of Oesophagostomum larvae in the gut mucosa63 in freshly infected individuals. Early immune responses are expected against parasitic antigens as well as tissue damage. Consequently, it is possible that changes in uNEO levels occur on a short timescale which we were unable to capture with our sampling regime. Similarly, the effects of SIV infection in experimentally infected rhesus and longtailed macaques were pronounced, but transient, with clearly elevated uNEO levels between 10–20 days after infection and subsequently moderately elevated levels26, with the changes expected in our experiment being smaller than those in an infection with SIV.
Fourth and most importantly, individuals in the study population are treated regularly and are thus infected repeatedly on a regular basis. Laboratory studies on immune-parasite interactions are often on first infections, which usually elicit strong immune responses94. Subsequent infections often lead to less pronounced immune response patterns95,96. Even in infections such as malaria in which NEO levels are expected to rise markedly, repeated infections have been demonstrated to attenuate the NEO response97. Given that for most host individuals, parasite burdens will be low while only a few show high parasite burdens98 and natural infections with GI parasites usually occur via low-dose trickle infections96, changes in the infection status may not necessarily lead to pronounced changes in the immune system balance of most hosts. Additionally, infections early in life, which are to be expected in our study population, have been shown to induce long lasting shifts of the immune system towards Th2 responses that are not influenced by repeated anthelmintic treatment99, a scenario that might well mimic the situation of our study population. Further studies using more frequent sampling and focussing on first reinfections in young individuals might help to evaluate whether NEO can be used as an indirect marker of GI parasites in nonhuman primates.
Conclusion
In accordance with previous studies on human and nonhuman primates, aged Barbary macaques of our study groups showed higher uNEO levels compared to younger adult individuals. This leads us to conclude that semi-free ranging Barbary macaques, presumably faced with a more natural range of pathogens than are laboratory nonhuman primates, show signs of immunosenescence which can be measured by uNEO levels. For these reasons, we argue that uNEO is a potential marker for studies on wildlife health, particularly for studies on the effects of aging, despite the lack of effect of parasite clearance on uNEO in our study subjects. We suggest future studies linking longitudinal data on uNEO levels with data on individuals’ survival and reproductive success. Including uNEO measurements might prove a valuable contribution to studies of aging, decreased immune function, morbidity and factors determining longevity and survival in wildlife.
Additional Information
How to cite this article: Müller, N. et al. Age, but not anthelmintic treatment, is associated with urinary neopterin levels in semi-free ranging Barbary macaques. Sci. Rep. 7, 41973; doi: 10.1038/srep41973 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Nunn, C. L. & Altizer, S. Infectious Diseases in Primates: Behavior, Ecology and Evolution (Oxford Series in Ecology and Evolution. Oxford University Press, 2006).
Tompkins, D. M., Dunn, A. M., Smith, M. J. & Telfer, S. Wildlife diseases: from individuals to ecosystems. Journal of Animal Ecology 80, 19–38 (2011).
Freeland, W. Pathogens and the evolution of primate sociality. Biotropica 8, 12–24 (1976).
Hill, A. V. The immunogenetics of human infectious diseases. Annual Review of Immunology 16, 593–617 (1998).
Karlsson, E. K., Kwiatkowski, D. P. & Sabeti, P. C. Natural selection and infectious disease in human populations. Nature Review Genetics 15, 379–393 (2014).
Buer, J. & Balling, R. Mice, microbes and models of infection. Nature Review Genetics 4, 195–205 (2003).
Gardner, M. B. & Luciw, P. A. Macaque models of human infectious disease. ILAR journal/National Research Council, Institute of Laboratory Animal Resources 49, doi: 10.1093/ilar.49.2.220 (2008).
Altizer, S. et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annual Review of Ecology and Systematics 34, 517–547 (2003).
Nussey, D. H., Froy, H., Lemaitre, J.-F., Gaillard, J.-M. & Austad, S. N. Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Research Reviews 12, 214–225 (2013).
Watson, R. L. et al. Cellular and humoral immunity in a wild mammal: Variation with age & sex and association with overwinter survival. Ecology and Evolution, doi: 10.1002/ece3.2584 (2016).
Jégo, M. et al. Haematological parameters do senesce in the wild: evidence from different populations of a long-lived mammal. Journal of Evolutionary Biology 27, 2745–2752 (2014).
MacIntosh, A. J. J. et al. Monkeys in the middle: Parasite transmission through the social network of a wild primate. PLoS ONE 7, doi: 10.1371/journal.pone.0051144 (2012).
Young, C., Majolo, B., Heistermann, M., Schülke, O. & Ostner, J. Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. Proceedings of the National Academy of Sciences of the United States of America 111, 18195–18200 (2014).
Muehlenbein, M. & Bribiescas, R. Testosterone-mediated immune functions and male life histories. American Journal of Human Biology 17, 527–558 (2005).
Muehlenbein, M. P. & Watts, D. P. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosocial Medicine 4, 21–21 (2010).
Maréchal, L., Semple, S., Majolo, B. & MacLarnon, A. Assessing the effects of tourist provisioning on the health of wild Barbary macaques in Morocco. PLoS ONE 11, doi: 10.1371/journal.pone.0155920 (2016).
Borg, C., Majolo, B., Qarro, M. & Semple, S. A Comparison of body size, coat condition and endoparasite diversity of wild Barbary macaques exposed to different levels of tourism. Anthrozoos 27, 49–63 (2014).
Archie, E. A., Altmann, J. & Alberts, S. C. Social status predicts wound healing in wild baboons. Proceedings of the National Academy of Sciences 109, 9017–9022 (2012).
Grueter, C. C., Deschner, T., Behringer, V., Fawcett, K. & Robbins, M. M. Socioecological correlates of energy balance using urinary C-peptide measurements in wild female mountain gorillas. Physiology & Behavior 127, 13–19 (2014).
Girard-Buttoz, C. et al. Urinary C-peptide measurement as a marker of nutritional status in macaques. PLoS ONE 6, doi: 10.1371/journal.pone.0018042 (2011).
Cristóbal-Azkarate, J., Maréchal, L., Semple, S., Majolo, B. & MacLarnon, A. Metabolic strategies in wild male Barbary macaques: evidence from faecal measurement of thyroid hormone. Biology Letters 12, doi: 10.1098/rsbl.2016.0168 (2016).
Schaebs, F. S., Wolf, T. E., Behringer, V. & Deschner, T. Fecal thyroid hormones allow for the noninvasive monitoring of energy intake in capuchin monkeys. Journal of Endocrinology 231, 1–10 (2016).
Graham, A. L. et al. Fitness Correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330, 662–665 (2010).
Ezenwa, V. O., Etienne, R. S., Luikart, G., Beja-Pereira, A. & Jolles, A. E. Hidden consequences of living in a wormy world: Nematode-induced immune suppression facilitates Tuberculosis invasion in African buffalo. The American Naturalist 176, 613–624 (2010).
Abbott, D. H. et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior 43, 67–82 (2003).
Higham, J. P. et al. Evaluating noninvasive markers of nonhuman primate immune activation and inflammation. American Journal of Physical Anthropology 158, 673–684 (2015).
Heistermann, M. & Higham, J. P. Urinary neopterin, a non-invasive marker of mammalian cellular immune activation, is highly stable under field conditions. Scientific Reports 5, 16308, doi: 10.1038/srep16308 (2015).
Murr, C., Widner, B., Wirleitner, B. & Fuchs, D. Neopterin as a marker for immune system activation. Current Drug Metabolism 3, 175–187 (2002).
Widner, B. et al. The importance of neopterin as a laboratory diagnostic marker of immune activation. Pteridines 10, 101–111 (1999).
Husain, N. et al. Neopterin concentration as an index of disease activity in Crohn’s disease and ulcerative colitis. Journal of Clinical Gastroenterology 47, 246–251 (2013).
Unal, B. et al. Serum neopterin as a prognostic indicator in patients with gastric carcinoma. Journal of Investigative Surgery 22, 419–425 (2009).
Breineková, K., Svoboda, M., Smutná, M. & Vorlová, L. Markers of acute stress in pigs. Physiological Research. 56, 323–329 (2007).
Berdowska, A. & Zwirska-Korczala, K. Neopterin measurement in clinical diagnosis. Journal of Clinical Pharmacy and Therapeutics 26, 319–329 (2001).
Aulitzky, W. E. et al. Comparison of serum neopterin levels and urinary neopterin excretion in renal allograft recipients. Clinical Nephrology 29, 248–252 (1988).
Fendrich, C. et al. Urinary neopterin concentrations in rhesus monkeys after infection with simian immunodeficiency virus (SIVmac 251). AIDS 3, 305–308 (1989).
Hawkley, L. C. & Cacioppo, J. T. Stress and the aging immune system. Brain, Behavior, and Immunity 18, 114–119 (2004).
Goodwin, K., Viboud, C. & Simonsen, L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24, doi: 10.1016/j.vaccine.2005.08.105 (2006).
Li, H., Manwani, B. & Leng, S. X. Frailty, inflammation, and immunity. Aging and Disease 2, 466–473 (2014).
Faria, A. M. C. et al. Variation rhythms of lymphocyte subsets during healthy aging. Neuroimmunomodulation 15, 365–379 (2008).
Deeks, S. G. HIV infection, inflammation, immunosenescence, and aging. Annual Review of Medicine 62, 141–155 (2011).
Hearps, A. C. et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11, 867–875 (2012).
Solana, R. et al. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Seminars in Immunology 24, 331–341 (2012).
Bauer, M. E., Jeckel, C. M. M. & Luz, C. The role of stress factors during aging of the immune system. Annals of the New York Academy of Sciences 1153, 139–152 (2009).
Frick, B., Schroecksnadel, K., Neurauter, G., Leblhuber, F. & Fuchs, D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clinical Biochemistry 37, 684–687 (2004).
Murr, C. et al. Increased neopterin concentration in older age coincides with decline of CD28 + CD45RA + T-cells. Pteridines 15, 170–174 (2004).
Anderson, R. M. & Colman, R. J. Prospects and perspectives in primate aging research. Antioxidants & Redox Signaling 14, 203–205 (2011).
Messaoudi, I., Estep, R., Robinson, B. & Wong, S. W. Nonhuman primate models of human immunology. Antioxidants & Redox Signaling 14, 261–273 (2011).
Meyer, C., Kerns, A., Haberthur, K. & Messaoudi, I. Improving immunity in the elderly: current and future lessons from nonhuman primate models. Age 34, 1157–1168 (2012).
Weng, N.-P. Aging of the immune system: How much can the adaptive immune system adapt? Immunity 24, 495–499 (2006).
Didier, E. S., Sugimoto, C., Bowers, L. C., Khan, I. A. & Kuroda, M. J. Immune correlates of aging in outdoor-housed captive rhesus macaques (Macaca mulatta). Immunity & Ageing 9, 1–15 (2012).
Haberthur, K., Engelman, F., Barron, A. & Messaoudi, I. Immune senescence in aged nonhuman primates. Experimental Gerontology 45, doi: 10.1016/j.exger.2010.06.001 (2010).
Huffman, M. A. & Chapman, C. A. Primate Parasite Ecology: The Dynamics and Study of Host-Parasite relationships (ed Cambridge University Press) (Cambridge, 2009).
Ferrari, N., Cattadori, I. M., Nespereira, J., Rizzoli, A. & Hudson, P. J. The role of host sex in parasite dynamics: field experiments on the yellow-necked mouse Apodemus flavicollis. Ecology Letters 7, 88–94 (2004).
Ezenwa, V. O. & Jolles, A. E. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science 347, 175–177 (2015).
Long, K. Z. & Nanthakumar, N. Energetic and nutritional regulation of the adaptive immune response and trade-offs in ecological immunology. American Journal of Human Biology 16, 499–507 (2004).
Carvalho, L. et al. Review series on helminths, immune modulation and the hygiene hypothesis: Mechanisms underlying helminth modulation of dendritic cell function. Immunology 126, 28–34 (2009).
Derting, Terry L. & Compton, S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiological and Biochemical Zoology 76, 744–752 (2003).
Martin, L. B., Scheuerlein, A. & Wikelski, M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proceedings of the Royal Society of London . Series B: Biological Sciences 270, 153–158 (2003).
Spencer, M. E. et al. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 65A, 858–865 (2010).
Ledochowski, M., Murr, C., Widner, B. & Fuchs, D. Association between insulin resistance, body mass and neopterin concentrations. Clinica Chimica Acta 282, 115–123 (1999).
Deschner, T., Kratzsch, J. & Hohmann, G. Urinary C-peptide as a method for monitoring body mass changes in captive bonobos (Pan paniscus). Hormones and Behavior 54, 620–626 (2008).
de Turckheim, G. & Merz, E. in The Barbary macaque: a case study in conservation (ed J. E. Fa ) 241–261 (Plenum Press, 1984).
Dash, K. M. The life cycle of Oesophagostomum columbianum (Curtice, 1890) in sheep. International Journal for Parasitology 3, 843–851 (1973).
Danish, L. M., Heistermann, M., Agil, M. & Engelhardt, A. Validation of a novel collection device for non-invasive urine sampling from free-ranging animals. PLoS ONE 10, doi: 10.1371/journal.pone.0142051 (2015).
Higham, J. P., Girard-Buttoz, C. d., Engelhardt, A. & Heistermann, M. Urinary C-peptide of insulin as a non-invasive marker of nutritional status: some practicalities. PLoS ONE 6, doi: 10.137/journal.pone.0022398 (2011).
Gillespie, T. R. Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. International Journal of Primatology 27, 1129–1143 (2006).
Chandra, R. K. Nutrition and the immune system: an introduction. The American Journal of Clinical Nutrition 66, 460S–463S (1997).
Emery Thompson, M., Muller, M. N. & Wrangham, R. W. Technical note: Variation in muscle mass in wild chimpanzees: Application of a modified urinary creatinine method. American Journal of Physical Anthropology 149, 622–627 (2012).
Miller, R. C. et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clinical Chemistry 50, 924–932 (2004).
R development core team. A language and environment for statistical computing. (R foundation for statistical computing, 2011).
Capuron, L. et al. Activated immune system and inflammation in healthy ageing: Relevance for tryptophan and neopterin metabolism. Current Pharmaceutical Design 20, 6048–6057 (2014).
Schennach, H. et al. Factors influencing serum neopterin concentrations in a population of blood donors. Clinical Chemistry 48, 643–645 (2002).
Diamondstone, L. S. et al. Factors influencing serum neopterin and ß2-microglobulin levels in a healthy diverse population. Journal of Clinical Immunology 14, 368–374 (1994).
Reibnegger, G. et al. Approach to define “normal aging” in man. Immune function, serum lipids, lipoproteins and neopterin levels. Mechanisms of Ageing and Development 46, 67–82 (1988).
Werner, E. R. et al. Determination of neopterin in serum and urine. Clinical Chemistry 33, 62–66 (1987).
Shintaku, H., Isshiki, G., Hase, Y., Tsuruhara, T. & Oura, T. Normal pterin values in urine and serum in neonates and its age-related change throughout life. Journal of Inherited Metabolic Disease 5, 241–242 (1982).
Arshadi, D. et al. Plasma level of neopterin as a marker of disease activity in treated rheumatoid arthritis patients: Association with gender, disease activity and anti-CCP antibody. International Immunopharmacology 17, 763–767 (2013).
Parker, D. C. et al. Plasma neopterin level as a marker of peripheral immune activation in amnestic mild cognitive impairment and Alzheimer’s disease. International Journal of Geriatric Psychiatry 28, 149–154 (2013).
Solichová, D., Melichar, B., Svobodová, I., Bláha, V. & Zadák, Z. Fluorescence analysis of antioxidant vitamins and neopterin in nonagenarians. Biomedical Chromatography, 117–118 (1999).
Čičin-Šain, L. et al. Loss of naive T-cells and repertoire constriction predict poor response to vaccination in old primates. Journal of immunology (Baltimore, Md.: 1950) 184, 6739–6745 (2010).
Elenkov, I. J. & Chrousos, G. P. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends in Endocrinology and Metabolism 10, 359–368 (1999).
Steel, C., Lujan-Trangay, A., Gonzalez-Peralta, C., Zea-Flores, G. & Nutman, T. B. Transient changes in cytokine profiles following ivermectin treatment of onchocerciasis. Journal of Infectious Diseases 170, 962–970 (1994).
Sajid, M. S. et al. Effect of ivermectin on the cellular and humoral immune responses of rabbits. Life Sciences 80, 1966–1970 (2007).
López-Olvera, J. R., Höfle, U., Vicente, J., Fernández-de-Mera, I. G. & Gortázar, C. Effects of parasitic helminths and ivermectin treatment on clinical parameters in the European wild boar (Sus scrofa). Parasitology Research 98, 582–587 (2006).
Stankiewicz, M. et al. Influence of ivermectin on cellular and humoral immune responses of lambs. Veterinary Immunology and Immunopathology 44, 347–358 (1995).
Uhlíř, J. & Volf, P. Ivermectin: its effect on the immune system of rabbits and rats infested with ectoparasites. Veterinary Immunology and Immunopathology 34, 325–336 (1992).
Pit, D., Polderman, A., Baeta, S., Schulz-Key, H. & Soboslay, P. Parasite-specific antibody and cellular immune responses in humans infected with Necator americanus and Oesophagostomum bifurcum . Parasitology Research 87, 722–729 (2001).
Andreasen, A. et al. Immune and inflammatory responses in pigs infected with Trichuris suis and Oesophagostomum dentatum . Veterinary Parasitology 207, 249–258 (2015).
Else, K. J. & Grencis, R. K. Cellular immune-responses to the murine nematode parasite Trichuris-muris.1. Differential cytokine production during acute or chronic infection. Immunology 72, 508–513 (1991).
Hewitson, J. P., Grainger, J. R. & Maizels, R. M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Molecular and Biochemical Parasitology 167, 1–11 (2009).
Hsieh, G. C.-F. et al. A secreted protein from the human hookworm Necator americanus binds selectively to NK cells and induces IFN-γ production. The Journal of Immunology 173, 2699–2704 (2004).
Grencis, R. K. & Entwistle, G. M. Production of an interferon-gamma homologue by an intestinal nematode: functionally significant or interesting artefact? Parasitology 115, 101–105 (1997).
Hotez. P. J. et al. Hookworm infection. New England Journal of Medicine 351, 799–807 (2004).
Reber, L. L., Sibilano, R., Mukai, K. & Galli, S. J. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal immunology 8, 444–463 (2015).
Maizels, R. M. & Yazdanbakhsh, M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Reviews Immunology 3, 733–744 (2003).
Grencis, R. K., Humphreys, N. E. & Bancroft, A. J. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunological Reviews 260, 183–205 (2014).
Brown, A. E., Webster, H. K., Teja-Isavadharm, P. & Keeratithakul, D. Macrophage activation in falciparum malaria as measured by neopterin and interferon-gamma. Clinical & Experimental Immunology 82, 97–101 (1990).
Hawley, D. M. & Altizer, S. M. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Functional Ecology 25, 48–60 (2011).
Wright, V. J. et al. Early exposure of infants to GI nematodes induces Th2 dominant immune responses which are unaffected by periodic anthelminthic treatment. PLoS Neglected Tropical Diseases 3, doi: 10.1371/journal.pntd.0000433 (2009).
Acknowledgements
We thank Ellen Merz and Roland Hilgartner for permission to conduct the study, Mamisolo Hilgartner and Bernhard Stark for help with anthelmintic treatment, the Affenberg staff for support, Andrea Heistermann for assisting in uNEO and uCP measurements, Holger Sennhenn-Reulen for help with statistical analyses, our Master students, Nicole Paschek, Navina Lilie and Zina Maria Morbach and our field assistants Alice Bouchard, Breanne Boughan, Fabrizio del’Anna, Frauke Gärtner, Cassandra Gaylor, Georg Messerer, Lola Rivoal, Graciela Sanguino, Juliane Schindler, Andrea Schick, Aaron Skinner, Natalie Weil, Louise Wilson and Rachel Voyt. We thank the members of the DFG-research group FOR 2136 “Sociality and health in primates” for fruitful discussions, in particular Cornelia Kraus and Sascha Knauf. We are grateful to two anonymous reviewers for constructive comments on the manuscript. This research was funded by the Deutsche Forschungsgemeinschaft (Os 201/6-1). NM also acknowledges funds from the German National Academic Foundation (Studienstiftung des deutschen Volkes) and the Gerhard-Mazurczak-Foundation.
Author information
Authors and Affiliations
Contributions
J.O., O.S. and N.M. designed the study. N.M. collected the data. N.M. and C.S. performed the parasite analysis. M.H. performed the uNEO and uCP analysis. N.M. took the lead in paper writing and created the figures, all other authors contributed to the writing. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Müller, N., Heistermann, M., Strube, C. et al. Age, but not anthelmintic treatment, is associated with urinary neopterin levels in semi-free ranging Barbary macaques. Sci Rep 7, 41973 (2017). https://doi.org/10.1038/srep41973
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41973
This article is cited by
-
Neopterin Levels in Bonobos Vary Seasonally and Reflect Symptomatic Respiratory Infections
EcoHealth (2023)
-
Urinary neopterin reflects immunological variation associated with age, helminth parasitism, and the microbiome in a wild primate
Scientific Reports (2022)
-
Urinary neopterin of wild chimpanzees indicates that cell-mediated immune activity varies by age, sex, and female reproductive status
Scientific Reports (2021)
-
Social and sexual behaviors predict immune system activation, but not adrenocortical activation, in male rhesus macaques
Behavioral Ecology and Sociobiology (2021)
-
Detection of neopterin in the urine of captive and wild platyrrhines
BMC Zoology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.