Abstract
Genome wide association studies (GWAS) have identified single-nucleotide polymorphisms (SNPs) that are associated with fasting plasma glucose (FPG) in adult European populations. The contribution of these SNPs to FPG in non-Europeans and children is unclear. We studied the association of 15 GWAS SNPs and a genotype score (GS) with FPG and 7 metabolic traits in 1,421 Mexican children and adolescents from Mexico City. Genotyping of the 15 SNPs was performed using TaqMan Open Array. We used multivariate linear regression models adjusted for age, sex, body mass index standard deviation score, and recruitment center. We identified significant associations between 3 SNPs (G6PC2 (rs560887), GCKR (rs1260326), MTNR1B (rs10830963)), the GS and FPG level. The FPG risk alleles of 11 out of the 15 SNPs (73.3%) displayed significant or non-significant beta values for FPG directionally consistent with those reported in adult European GWAS. The risk allele frequencies for 11 of 15 (73.3%) SNPs differed significantly in Mexican children and adolescents compared to European adults from the 1000G Project, but no significant enrichment in FPG risk alleles was observed in the Mexican population. Our data support a partial transferability of European GWAS FPG association signals in children and adolescents from the admixed Mexican population.
Similar content being viewed by others
Introduction
In 2011, 366 million people worldwide were diagnosed with diabetes, of which more than 90% were Type 2 Diabetes (T2D), and the prevalence may reach 552 million people by 20301. T2D can be diagnosed based on one of several criteria and clinical symptoms of diabetes include polyuria, polydipsia, weight loss, blurred vision and fatigue2. Random blood glucose, oral glucose tolerance or glycosylated haemoglobin are also commonly used to diagnose T2D. However, fasting plasma glucose (FPG) is the most widely used biochemical tool to diagnose T2D in clinical setting (provisional diabetes diagnosis if FPG ≥ 7.0 mmol/L). FPG values predict incident cardiovascular outcomes in normoglycaemic and dysglycaemic subjects and is therefore an important biological marker in prevention of T2D and its complications3. Understanding the underlying physiology of FPG regulation is essential to improving our knowledge of T2D pathophysiology. Variations in FPG could stem from either genetic or environmental factors. Heritability estimates for FPG range from 38% to 51%, based on findings in twin studies4,5. Since 2008, GWAS have identified 53 FPG-associated loci mostly in European adult populations6,7. Nine of these FPG-associated loci were shown to interact with body mass index (BMI)6,8. Only a partial overlap was observed between GWAS loci associated with FPG level and T2D9. This supports the view that the genetic dissection of both extremes of the disease and intermediary quantitative traits is necessary for the understanding of glucose homeostasis9. FPG-associated loci identified in European populations were in part replicated in various ethnic groups10,11,12. Only one study to date have assessed the contribution of GWAS identified European adult FPG-associated loci in a youth population13. In this study, Barker et al. showed that 9 GWAS identified loci were associated with FPG levels in over 6,000 European children and adolescents with effect sizes comparable to adults13. However, the contribution of these GWAS identified loci to FPG level in non-European children has not been investigated so far.
The high obesity and dysglycemia predispositions of specific ethnic groups may be related to their past evolutive history14. James Neel suggested that the ‘thrifty’ genes would have been advantageous in the past, because it would allow for accumulation of fat quickly during times of abundance. This thrifty genotype would then increase individual survival during times of food scarcity. However, in modern societies with a constant abundance of food, this genotype efficiently prepares individuals for a hypothetical famine and predisposes to obesity and dysglycemia14. The Mexican population may be illustrative of this paradigm, as the prevalence of obesity and T2D are among the highest in the world15. In addition, the unique history of the Mexican population led to a complex admixed population, combining genomes of European, Native South-American and African ancestry with divergent susceptibility to metabolic diseases16.
Here we assessed the contribution of 15 loci initially associated with FPG in European adults in a population of 1,421 Mexican children and adolescents from Mexico City.
Results
Characteristics of the Mexican children population
The main anthropometric and biological characteristics of the 1,421 Mexican children are summarized in Table 1. Of the 1,421 Mexican children, 34 (2.4%) had FPG ≥ 5.6 mmol/L and 1 (0.07%) had FPG ≥ 7.0 mmol/L. In addition, 223 (15.8%) children presented clinical measures that were consistent metabolic syndrome as defined by the International Diabetes Federation (IDF) consensus of 200717.
Association of SNPs with fasting plasma glucose in Mexican children
Of the 15 SNPs tested, MTNR1B rs10830963 (β = 0.11 ± 0.04, P = 0.0091), G6PC2 rs560887 (β = 0.12 ± 0.06, P = 0.049), and GCKR rs1260326 (β = 0.07 ± 0.04, P = 0.049) were significantly associated with FPG levels in Mexican children (Table 2) in a consistent direction of effect as those seen in initial GWAS reports in European adult populations (See Supplementary Table S2). The FPG risk alleles of a majority of SNPs (11 out of the 15 SNPs, 73.3%) had beta values for FPG directionally consistent with those reported in adult European GWAS. The genotype score (GS) was significantly associated with FPG in our population of Mexican children (β = 0.03 ± 0.01, P = 0.012).
Association between genetic variant of fasting plasma glucose and continuous metabolic traits
The associations between the 15 genetic variants and 7 continuous metabolic traits are reported in Supplementary Table S1. Seven SNPs displayed a nominal evidence of association: DGKB/TMEM195 rs2191349 with standard deviation score (SDS) BMI (β = 0.085 ± 0.038, P = 0.025) and with SDS-waist-to-hip ratio (SDS-WHR) (β = 0.044 ± 0.016, P = 0.008); ADCY5 rs11708067 with SDS-WHR (β = −0.035 ± 0.016, P = 0.034); FADS1 rs174550 with triglyceride (TG) (β = −0.085 ± 0.042, P = 0.045), with total cholesterol (Total-c) (β = 0.093 ± 0.039, P = 0.017) and LDL-cholesterol (LDL-c) (β = 0.145 ± 0.041, P = 0.00048); GCKR rs1260326 with TG (β = −0.092 ± 0.039, P = 0.018) and Total-c (β = −0.108 ± 0.003, P = 0.003); GLIS3 rs7034200 with TG (β = −0.075 ± 0.036, P = 0.035); MTNR1B rs10830963 with HDL-cholesterol (HDL-c) (β = −0.109 ± 0.042, P = 0.010); and SLC30A8 with fasting plasma insulin (FPI) level (β = 0.96 ± 0.044, P = 0.027). The GS showed a nominal association with lower TG (β = −0.024 ± 0.011, P = 0.035) and higher FPI levels (β = 0.026 ± 0.012, P = 0.033). After appropriate Bonferroni correction, no association of SNPs with metabolic traits remained significant (P < 4.5 × 10−4).
Gene by gene interaction
We examined gene x gene interactions by testing pairwise interactions between the 15 SNPs on FPG (all possible combinations). While some interactions appeared nominally significant none survived Bonferroni correction. Interestingly, the SNP rs11708067 in ADCY5 displayed nominal evidence of interaction with SNPs in/near 4 different genes (FADS1, G6PC2, DGKB/TMEM195, GLIS3) (See Supplementary Table S3).
Allele frequency
Allele frequencies of the SNPs in this cohort were compared with those from European adults in the 1000 Genomes Project (1000G). The risk allele frequencies (RAFs) for 11 of 15 (73.3%) SNPs differed significantly in Mexican children compared to Europeans (P < 3.3 × 10−3) (Table 3). Of these, 6 were enriched and 5 were depleted in Mexican children and adolescents compared to Europeans (binomial test P = 1). Allele frequencies were also compared between Mexican children and Mexican adults from the 1000G reference panel (See Supplementary Table S4). The RAF of 1 SNP out of 15 was significantly enriched in Mexican children and adolescents compared to Mexican adults.
Discussion
The growing rates of obesity, pre-diabetes and T2D are becoming a major health concern in the Mexican population18. The high lifetime risk of developing diabetes among Hispanic maybe explained by lifestyle and biological risk factors, including genetic susceptibility19,20. This study is the first to assess the transferability of previously identified European FPG SNPs in a population of Mexican children and adolescents. Our data support a partial transferability of FPG association signals in the admixed Mexican population. Three out of the 15 selected FPG SNPs (G6PC2 rs560887, GCKR rs1260326 and MTNRB1 rs10830963) were significantly associated with FPG in Mexican children. These SNPs are among the strongest genetic contributors previously identified in GWAS of adult populations of European ancestry21,22,23,24. Overall, the direction of effect of three-quarter of the FPG SNPs investigated is consistent with what was previously reported in GWAS21,24. This is consistent with data in children and adolescents of European ancestry where 56% of SNPs originally associated with FPG in adults replicated13. In addition, G6PC2 rs560887 and MTNRB1 rs10830963 SNPs are associated with FPG both in Mexican children and in European children and adolescents13. The GS also show a significant association with elevated FPG in Mexican children. Altogether, our data support a partial transferability of adult European FPG SNPs in Mexican children and adolescents. This observation is consistent with the fact that part of the Mexican genome is from European descent25,26. While ethnic-specific linkage disequilibrium structure may contribute to between population heterogeneity, it is likely to play a smaller role as the direction of effect of risk alleles is consistent with that in European adults for a majority of the SNPs. Alternatively, gene x gene, gene x lifestyle, gene x age interactions or epigenetic differences may contribute to between population heterogeneity27,28. Stressing the fact that FPG levels are not influenced by the same factors in children and adults is also important29. Our findings confirm that SNPs previously identified in specific ethnic groups are relevant candidates for association analyses in other populations11,12,30,31,32.
The association between the FPG SNPs and other metabolic traits was also investigated. These included SDS-BMI, SDS-WHR, TG, total-c, HDL-c, LDL-c and FPI. To ensure that the associations detected were not the result of indirect effects mediated by an increase in FPG, the models were adjusted accordingly. The results indicate a nominally significant association between the C-allele rs1260326 of GCKR and LDL-c, total-c and TG. This is consistent with the inverse association observed between rs1260326, lipids and FPG in European adults33. Similarly, the nominally significant association of FADS1 rs174550 with TG and LDL-c has been previously reported in adult populations of European descent21,34. Thus our results, if confirmed, may suggest that the pleiotropic associations initially described in European adults are also transferable to Mexican children and adolescents.
When we examined the allele distribution between Mexican children and adolescents and European adults from 1000G, three-quarter of the 15 FPG SNPs tested displayed significant differences in the RAFs. There was no evidence of FPG increasing allele enrichment in Mexican children (binomial test, P = 1.00). If confirmed, the differences of RAF observed between European and Mexican populations most likely result from the unique history of the admixed Mexican population rather than local natural selection pressures. Overall, no major differences were observed for the FPG effect alleles between Mexican children and adolescents and Mexican adults from the 1000G. These data may suggest that the FPG SNPs have no major effect on longevity35.
We investigated gene x gene interactions and ADCY5 showed a nominal interaction with 4 other gene variants in/near DGKB/TMEM195, FADS1, G6PC2 and GLIS3. The adenylate cyclase 5 enzyme regulates the increase of Ca2+ in response to increased blood glucose levels. ADCY5 catalyzes the formation of the signaling molecule cAMP in response to G-protein signaling and mediates signaling downstream of the adrenergic receptor beta 1. Interestingly, we found that ADCY5 may potentially interact directly with at least 10 proteins, including several well-established FPG loci such as MTNR1B or GIPR (http://genomics.senescence.info/genes/human.html). The ability of ADCY5 to interact with other proteins may explain why gene x gene interactions at different loci are observed36. This is in agreement with the quantitative genetics model of fluxes and metabolic pools37. Inactivation of ADCY5 in mice impacts multiple pleiotropic traits as diverse as oxidative stress, energy balance, bone and cardiovascular health and longevity36,38,39. Furthermore, SNPs in ADCY5 have shown robust associations with various traits such as birth weight, FPG, 2-hour glucose post OGTT and T2D21,40,41,42,43. Altogether, these data suggest that ADCY5 may be a key regulator of metabolism, even though the mechanisms underlying possible effect at the level of pancreatic β-cells remain unclear44. Although none of the genes that showed nominal interactions with ADCY5 survived the Bonferroni adjustment, possibly due to our modest sample size (Supplementary Figure S2), further investigation of these genes could lead to new insight on the interactions between FPG loci in Mexican and other populations.
Our study has several strengths. We assessed for the first time the transferability of FPG SNPs previously identified in European adults in a population of Mexican children and adolescents. The recruitment of Mexican children was limited to one city, which restricts the range of environmental exposures and increases the power to identify genetic associations with multifactorial traits. By testing the possibility of gene pleiotropy, gene epistasis or local natural selection signatures, our study goes beyond traditional post-GWAS replication studies. However, our study is not without its caveats. We acknowledge that our sample had a modest power to detect main genetic effects and gene x gene interactions (Supplementary Figure S2). Our list of SNPs (N = 15) is not the most up to date as 45 SNPs have been conclusively associated with FPG in European adult populations so far. We did not adjust for population substructure within our sample, which may increase the risk of false positive association45. The cross-sectional nature of this study precludes causal inferences to be made about the associations described here.
In summary, our findings suggest a partial transferability of FPG SNPs identified in European adult populations in the admixed population of Mexican children and adolescents. Our data confirm the high trans-ethnic replicability of GWAS results and the value of performing GWAS in diverse ethnic groups to elucidate the molecular underpinnings of dysglycemia32. Our results support that a subset of SNPs modulate FPG levels early in life46. This may help to design and implement early personalized prevention strategies against dysglycemia and its complications in the future47.
Methods
Study population
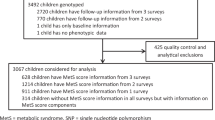
A total of 1,559 children between the ages of 5 and 17 were randomly selected to participate in a cross-sectional study from four areas in Mexico City at the Primary Care Unit of the National Mexican Social Security Institute (Cuauhtémoc West, Independencia South, Nezahualcóyotl Est and Morelos North area). Recruitment was done in collaboration with local public schools. The study started in July 2011 and is still ongoing. A trained pediatrician performed all the anthropometric measurements. Blood samples were collected for biochemical measurements and DNA extraction. Children who had diagnosis of infectious disease, gastrointestinal disorders, administration of antimicrobial agents (within 6 months previous to study), incomplete questionnaires or biological samples were excluded. The child’s assent and written informed consent from the parents/guardians was obtained prior to enrolment into the study. The study protocol was approved by the Mexican Social Security Institute National Committee and the Ethical Committee Board. All procedures were conducted in accordance with the Declaration of Helsinki48.
Anthropometric and Biochemical Measurements
Participants were scheduled for clinical laboratory evaluation following a 12 hour overnight fasting. All participants were weighed using a digital scale (Seca, Hamburg, Germany). Height was measured with a portable stadiometer (Seca 225, Hamburg, Germany). Height, weight and BMI, calculated as weight (kg)/height (m)2, were converted to age- and sex- adjusted standard deviation scores (SDS-Height, SDS-Weight and SDS-BMI, respectively) using the LMS method according to guidelines from the centers for disease control (CDC)49,50. Waist circumference (WC) and hip circumference (HC) were measured at the midpoint between the lowest rib and the iliac crest at the top of the iliac crest respectively, after a normal exhalation with children in the standing position. The WC and the waist to hip ratio (WHR) were also converted to age- and sex- adjusted standard deviation scores (SDS-WC and SDS-WHR, respectively) using the LMS method and growth charts based on US National Health and Nutrition Survey, cycle III (NHANES III)51. Systolic and diastolic blood pressure (SBP and DBP) were measured using a mercurial sphygmomanometer (ALPK2, Tokyo, Japan). Blood pressure readings were taken for each participant twice on the right arm in a sitting position with 5 minutes rest between each measurement and the mean of the two readings was determined. Age- and sex- adjusted standard deviations scores for SBP and DBP (SDS-SBP and SDS-DBP) were calculated using methods specified by the fourth report from the National High Blood Pressure Education Program (NHBPEP) in children and adolescents52. Blood samples were obtained following a 12 hour fast and were analyzed for FPG, total-c, HDL-c, LDL-c and triglycerides TG using the ILab 350 Clinical Chemistry System (Instrumentation Laboratory IL. Barcelona Spain). FPI (IU) was measured by chemiluminescence (IMMULITE, Siemens, USA). Metabolic syndrome was assigned based on the IDF consensus definition of the metabolic syndrome in children and adolescents17.
Genotyping
Genomic DNA was extracted from peripheral blood with cells using the FLEX STAR Autogen platform (Holliston, Massachusetts US). One hundred twenty-eight SNPs that have been previously associated with metabolic traits were genotyped using the TaqMan OpenArray Real-Time PCR System (Life Technologies, Carlsbad, US), following the manufacturer’s instructions. Following quality control, 1,421 participants with both genotype and clinical data were retained for further analysis53. A list of SNPs that reached genome-wide significance (P < 5 × 10−8) with FPG level in adult populations of European ancestry was established. Three different strategies were used to optimize the SNP selection procedure using a key word search on i) the National Human Genome Research Institute (NHGRI) GWAS Catalog (www.genome.gov/gwastudies/) ii) the HuGE Navigator GWAS Integrator (www.hugenavigator.net/HuGENavigator/gWAHitStartPage.do) iii) the PubMed database (www.ncbi.nlm.nih.gov/pubmed). Using this strategy, 16 independent SNPs were selected in October 2012. One SNP was not successfully genotyped (rs11071657 near C2CD4B) whereas 15 SNPs passed the quality control criteria: rs560887 in G6PC2, rs1260326 in GCKR, rs4607517 near GCK, rs10830963 in MTNR1B, rs2191349 near DGKB-TMEM195, rs11708067 in ADCY5, rs7944584 in MADD, rs10885122 near ADRA2A, rs174550 in FADS1, rs11605924 in CRY2, rs11920090 in SLC2A2, rs7034200 in GLIS3, rs340874 near PROX1, rs13266634 in SLC30A8, rs7903146 in TCF7L2. We did not observe significant deviation from Hardy-Weinberg Equilibrium (HWE) (all P ≥ 0.0033) for the 15 SNPs and the average call rate was 97.59% for the 15 SNPs (See Supplementary Table S2). Individuals with greater than 10% missing genotypes were excluded from the analysis.
Statistical Analyses
Allele frequencies in Europeans (N = 503) and Mexican adults (N = 64) were obtained from 1000G using Ensembl and compared to allele frequencies in Mexican children using chi-square tests as previously described53,54. Risk allele frequencies (RAF) were calculated using the FPG increasing allele reported in European GWAS (Table 3). Non-biological outlier data were discarded using a Cook’s distance test followed by an expert verification. Based on Shapiro-Wilk test (Supplementary Table S5), all the traits of interest deviated significantly from normality. Hence, rank based inverse normal transformations were applied wherever substantial deviations from normality were observed (See Supplementary Figure S1). Rank transformations corrected the lack of normality for all traits (Supplementary Table S5). Single SNP analyses were performed under the additive model, and the previously identified FPG increasing alleles for each of the 15 SNPs were used as the risk allele for the analyses. The association of SNPs/GS with FPG was assessed using linear regression models adjusted for age, sex, BMI and recruitment center. The association tests between SNPs/GS and additional metabolic traits were further adjusted for FPG to ensure that effects of SNPs were not the result of indirect associations with FPG (mediation versus pleiotropy). The genotype score (GS) was calculated by summing the alleles of the 15 FPG-associated SNPs so that the score ranged from 0 to 30. Since weighting has been shown to have no major impact on the overall GS55, an un-weighted GS was used for these analyses. We performed imputations for the missing genotypic values as previously described56. The imputation was performed for each locus using the mean number of the FPG alleles successfully genotyped for all individuals. For assessing gene x gene interactions, all possible pair-wise interactions between SNPs and FPG were tested (15C2 = 105) using linear regression models adjusted for age, sex, BMI and recruitment center. Two-tailed P-values are presented in this manuscript and P < 0.05 were considered significant when testing the association of the 15 SNPs and the GS with FPG in the Mexican children population, given the high prior probability of association. However, when testing those SNPs for HWE (P < 0.0033), differences of RAF between Mexican children and European adults from 1000G (P < 0.0033), and Mexican children and Mexican adults (P < 0.0033), gene x gene interactions (P < 4.8 × 10−4), and associations with metabolic traits (P < 4.5 × 10−4), correction for multiple testing was applied. We followed the strategy reported previously by Ronald J Feise and considered independent Bonferroni corrections for each question asked57. Statistical analyses were performed using R version 3.1.2. HWE was tested using the HardyWeinberg package58, LD was determined using genetics package, rank-based inverse-normal transformations were conducted using GenABEL59. Power calculations were performed using QUANTO (version 1.2.4, University of Southern California, Los Angeles, CA, USA).
Additional Information
How to cite this article: Langlois, C. et al. Evaluating the transferability of 15 European-derived fasting plasma glucose SNPs in Mexican children and adolescents. Sci. Rep. 6, 36202; doi: 10.1038/srep36202 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Whiting, D. R., Guariguata, L., Weil, C. & Shaw, J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94, 311–321 (2011).
American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes care 36 Suppl 1, S67–S74, doi: 10.2337/dc13-S067 (2013).
Anand, S. S. et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. Eur J Prev Cardiol 19, 755–764, doi: 10.1177/1741826711409327 (2012).
Katoh, S. et al. Genetic and environmental effects on fasting and postchallenge plasma glucose and serum insulin values in Finnish twins. The Journal of clinical endocrinology and metabolism 90, 2642–2647, doi: 10.1210/jc.2004-2471 (2005).
Leslie, R. D. et al. Level of an advanced glycated end product is genetically determined: a study of normal twins. Diabetes 52, 2441–2444 (2003).
Scott, R. A. et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 44, 991–1005, doi: 10.1038/ng.2385 (2012).
Comuzzie, A. G. et al. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PloS one 7, e51954, doi: 10.1371/journal.pone.0051954 (2012).
Manning, A. K. et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 44, 659–669, doi: 10.1038/ng.2274 (2012).
Barker, A., Langenberg, C. & Wareham, N. J. Genetic determinants of glucose homeostasis. Best Pract Res Clin Endocrinol Metab 26, 159–170, doi: 10.1016/j.beem.2011.12.002 (2012).
Li, H. et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 55, 981–995, doi: 10.1007/s00125-011-2370-7 (2012).
Ramos, E. et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia 54, 783–788, doi: 10.1007/s00125-010-2002-7 (2011).
Fesinmeyer, M. D. et al. Genetic variants associated with fasting glucose and insulin concentrations in an ethnically diverse population: results from the Population Architecture using Genomics and Epidemiology (PAGE) study. BMC Med Genet 14, 98, doi: 10.1186/1471-2350-14-98 (2013).
Barker, A. Association of Genetic Loci With Glucose Levels in Childhood and Adolescence. doi: 10.2337/db10-1575/-/DC1 (2011).
Neel, J. V. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 14, 353–362 (1962).
Jimenez-Cruz, A. & Bacardi-Gascon, M. The fattening burden of type 2 diabetes on Mexicans: projections from early growth to adulthood. Diabetes care 27, 1213–1215 (2004).
Martinez-Cortes, G. et al. Admixture and population structure in Mexican-Mestizos based on paternal lineages. J Hum Genet 57, 568–574, doi: 10.1038/jhg.2012.67 (2012).
Zimmet, P. et al. The metabolic syndrome in children and adolescents–an IDF consensus report. Pediatric diabetes 8, 299–306 (2007).
Goran, M. I., Lane, C., Toledo-Corral, C. & Weigensberg, M. J. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes 57, 3007–3012, doi: 10.2337/db08-0445 (2008).
Narayan, K. M., Boyle, J. P., Thompson, T. J., Sorensen, S. W. & Williamson, D. F. Lifetime risk for diabetes mellitus in the United States. Jama 290, 1884–1890 (2003).
Ogden, C. L., Flegal, K. M., Carroll, M. D. & Johnson, C. L. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288, 1728–1732 (2002).
Dupuis, J. et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42, 105–116, doi: 10.1038/ng.520 (2010).
Prokopenko, I. et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet 41, 77–81 (2009).
Bouatia-Naji, N. et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 320, 1085–1088, doi: 10.1126/science.1156849 (2008).
Barker, A. et al. Association of genetic Loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes 60, 1805–1812, doi: 10.2337/db10-1575 (2011).
Klimentidis, Y. C., Miller, G. F. & Shriver, M. D. The relationship between European genetic admixture and body composition among Hispanics and Native Americans. Am J Hum Biol 21, 377–382 (2009).
Klimentidis, Y. C., Miller, G. F. & Shriver, M. D. Genetic admixture, self-reported ethnicity, self-estimated admixture, and skin pigmentation among Hispanics and Native Americans. Am J Phys Anthropol 138, 375–383, doi: 10.1002/ajpa.20945 (2009).
Li, A. & Meyre, D. Challenges in reproducibility of genetic association studies: lessons learned from the obesity field. International Journal of Obesity 37, 559–567 (2013).
Lu, Y. & Loos, R. J. Obesity genomics: assessing the transferability of susceptibility loci across diverse populations. Genome medicine 5, 1 (2013).
Zijlmans, W. C., van Kempen, A. A., Serlie, M. J. & Sauerwein, H. P. Glucose metabolism in children: influence of age, fasting, and infectious diseases. Metabolism 58, 1356–1365 (2009).
Takeuchi, F. et al. Common variants at the GCK, GCKR, G6PC2-ABCB11 and MTNR1B loci are associated with fasting glucose in two Asian populations. Diabetologia 53, 299–308, doi: 10.1007/s00125-009-1595-1 (2010).
Rasmussen-Torvik, L. J. et al. Fasting glucose GWAS candidate region analysis across ethnic groups in the Multiethnic Study of Atherosclerosis (MESA). Genet Epidemiol 36, 384–391, doi: 10.1002/gepi.21632 (2012).
Marigorta, U. M. & Navarro, A. High trans-ethnic replicability of GWAS results implies common causal variants. PLoS Genet 9, e1003566, doi: 10.1371/journal.pgen.1003566 (2013).
Vaxillaire, M. et al. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 57, 2253–2257 (2008).
Kathiresan, S. et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 41, 56–65, doi: 10.1038/ng.291 (2009).
Lewis, S. J. & Brunner, E. J. Methodological problems in genetic association studies of longevity–the apolipoprotein E gene as an example. Int J Epidemiol 33, 962–970, doi: 10.1093/ije/dyh214 (2004).
Yan, L. et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130, 247–258, doi: 10.1016/j.cell.2007.05.038 (2007).
Bost, B., Dillmann, C. & de Vienne, D. Fluxes and metabolic pools as model traits for quantitative genetics. I. The L-shaped distribution of gene effects. Genetics 153, 2001–2012 (1999).
De Lorenzo, M. S. et al. ‘Reduced malignancy as a mechanism for longevity in mice with adenylyl cyclase type 5 disruption’. Aging Cell 13, 102–110, doi: 10.1111/acel.12152 (2014).
Freathy, R. M. et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat Genet 42, 430–435, doi: 10.1038/ng.567 (2010).
Saxena, R. et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nature genetics 42, 142–148 (2010).
Andersson, E. et al. Type 2 diabetes risk alleles near ADCY5, CDKAL1 and HHEX-IDE are associated with reduced birthweight. Diabetologia 53, 1908–1916 (2010).
Barker, D. J. et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36, 62–67 (1993).
Freathy, R. M. et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nature genetics 42, 430–435 (2010).
Hodson, D. J. et al. ADCY5 couples glucose to insulin secretion in human islets. Diabetes 63, 3009–3021, doi: 10.2337/db13-1607 (2014).
Tian, C., Gregersen, P. K. & Seldin, M. F. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet 17, R143–R150 (2008).
Sohani, Z. N. et al. Risk Alleles in/near ADCY5, ADRA2A, CDKAL1, CDKN2A/B, GRB10, and TCF7L2 Elevate Plasma Glucose Levels at Birth and in Early Childhood: Results from the FAMILY Study. PLoS One 11, e0152107, doi: 10.1371/journal.pone.0152107 (2016).
Li, A. & Meyre, D. Jumping on the Train of Personalized Medicine: A Primer for Non-Geneticist Clinicians: Part 2. Fundamental Concepts in Genetic Epidemiology. Current Psychiatry Reviews, 10, 101–117 (2014).
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194, doi: 10.1001/jama.2013.281053 (2013).
Flegal, K. M. & Cole, T. J. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Report 63, 1–4 (2013).
de Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85, 660–667 (2007).
Sharma, A. K., Metzger, D. L., Daymont, C., Hadjiyannakis, S. & Rodd, C. J. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatric research (2015).
Pediatrics, A. A. o. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. Pediatrics 114, iv-iv (2004).
Abadi, A. et al. Assessing the effects of 35 European‐derived BMI‐associated SNPs in Mexican children. Obesity (2016).
Rouskas, K. et al. Common variants in FTO, MC4R, TMEM18, PRL, AIF1, and PCSK1 show evidence of association with adult obesity in the Greek population. Obesity (Silver Spring) 20, 389–395, doi: 10.1038/oby.2011.177 (2012).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 9, e1003348, doi: 10.1371/journal.pgen.1003348 (2013).
Robiou-du-Pont, S. et al. Contribution of 24 obesity-associated genetic variants to insulin resistance, pancreatic beta-cell function and type 2 diabetes risk in the French population. International journal of obesity 37, 980–985, doi: 10.1038/ijo.2012.175 (2013).
Feise, R. J. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2, 8 (2002).
Graffelman, J. Exploring Diallelic Genetic Markers: The HardyWeinberg Package. Journal of Statistical Software 64, 1–23 (2015).
Aulchenko, Y. S., Ripke, S., Isaacs, A. & van Duijn, C. M. GenABEL: an R library for genome-wide association analysis. Bioinformatics 23, 1294–1296, doi: 10.1093/bioinformatics/btm108 (2007).
Acknowledgements
We thank all the study participants and the reviewers for their helpful comments. We acknowledge Aïhua Li and Hudson Reddon for their technical assistance. DM is supported by a Tier 2 Canada Research Chair in Genetics of Obesity. This work was supported by Fundación IMSS A.C. and by the National Council of Science and Technology (CONACYT-México) with the grant SALUD-2013-C01-201471 (FONSEC SSA/IMSS/ISSSTE).
Author information
Authors and Affiliations
Contributions
C.L., J.P.-R., M.C. and D.M. designed the experiment. J.P.-R. and M.C. contributed to the recruitment of participants and the clinical aspects of the study. J.P.-R., F.S. and J.G.-Z. performed the DNA extraction and genotyping experiments. C.L., A.A., A.A., A.B.G. and D.M. prepared the dataset for analysis. C.L., A.A. and D.M. conducted statistical analyses. C.L., A.A., A.A., F.T.Y. and D.M. wrote the manuscript and prepared all tables and figures. J.P.-R., F.S., J.G.-Z., A.I.B.-G. and M.C. critically reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Langlois, C., Abadi, A., Peralta-Romero, J. et al. Evaluating the transferability of 15 European-derived fasting plasma glucose SNPs in Mexican children and adolescents. Sci Rep 6, 36202 (2016). https://doi.org/10.1038/srep36202
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36202
This article is cited by
-
Genome-wide landscape establishes novel association signals for metabolic traits in the Arab population
Human Genetics (2021)
-
Melatonin in type 2 diabetes mellitus and obesity
Nature Reviews Endocrinology (2019)
-
Adiponectin is associated with cardio-metabolic traits in Mexican children
Scientific Reports (2019)
-
Genetic contribution to waist-to-hip ratio in Mexican children and adolescents based on 12 loci validated in European adults
International Journal of Obesity (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.