Abstract
Soil microorganisms play a crucial role in the biogeochemical cycling of nutrient elements and maintaining soil health. We aimed to investigate the response of bacteria communities to organic farming over different crops (rice, tea and vegetable) along the middle and lower reaches of the Yangtze River of China. Compared with conventional farming, organic farming significantly increased soil nutrients, soil enzyme activities, and bacterial richness and diversity. A Venn diagram and principal component analysis revealed that the soils with 3 different crops under organic farming have more number and percent of shared OTUs (operational taxonomic units), and shared a highly similar microbial community structure. Under organic farming, several predominant guilds and major bacterial lineages (Rhizobiales, Thiotrichaceae, Micromonosporaceae, Desulfurellaceae and Myxococcales) contributing to nutrient (C, N, S and P) cycling were enriched, whereas the relative abundances of acid and alkali resistant microorganisms (Acidobacteriaceae and Sporolactobacillaceae) were increased under conventional farming practices. Our results indicated that, for all three crops, organic farming have a more stable microflora and the uniformity of the bacterial community structure. Organic agriculture significantly increased the abundance of some nutrition-related bacteria, while reducing some of the abundance of acid and alkali resistant bacteria.
Similar content being viewed by others
Introduction
Soil is the fundamental resource of an agricultural ecosystem. Overuse of agricultural chemicals, such as fertilizers and pesticides, in conventional agriculture and intensive human activities have caused serious soil degradation and accumulation of pesticides1. Organic farming systems are an alternative to conventional agriculture2 that minimize the impact of agricultural practices on soil quality and the environment3. Due to these advantages, the land area under organic agricultural management reached 43.1 million hectares by the end of 2013, with an annual increase of 14%. The productivity of organically farmed land was equivalent to that conventional farming but was subject to less chemical and energy inputs2, reduced nutrient losses4, and lower global warming potential5. Vegetable fields under organic production even produced yields equal to those under conventional production6,7. Evidence showed that organic farming provided more biodiversity than conventional farming8 with respect to birds, plants, butterflies, insects, invertebrates and microbes9,10,11,12.
Soil microorganisms play a crucial role in the biogeochemical cycling of nutrient elements and maintaining soil health13,14,15. Farming practices have been proven to influence the composition of soil bacterial communities. Agricultural management had complex and diverse effects on the soil microbiome16,17. Arriving at universally valid conclusions about organic and conventional farming systems is difficult because of the complexity of the soil microbial community and the limitations imposed by the resolution of analytical methods. Organic farming has influences on domain-specific biomass18, increases richness2, reduces dispersion19 and shifts the structure of the soil microbiota2,19. Fertilizer was considered to be the primary driving factor leading to the changes in soil microbiota. A significant difference was detected in the composition of bacterial genera between organic and conventional management systems20. Organically managed soils exhibit greater biological activity than conventionally managed soils2,21.
Community structure is essential for the ecological function of soil microbiota. The change in soil microbial diversity had a great impact on the stability of the soil ecosystem22,23; the relationships between the diversity of the microbial community and the function and stability of the community are very complex. Organic farming lands showed a tendency to suppress plant pathogens. Foliar diseases, such as stripe rust, powdery mildew, and snow mold of wheat, as well as soil-borne pathogens, were observed to be less severe in organic fields than in conventional fields24. An analysis of the fungal community by 454 pyrosequencing showed that organic farms had a slightly higher diversity and evenness with respect to the microbial community compared with conventional farms. The relative abundance of some potato fungal pathogens was less in organic farms25. Aureobasidium pullulans was considered to contribute greatly to the control of pathogenic fungi and to the better taste of organically produced wine26. Plant growth-promoting bacteria belonging to the Burkholeria, Stenotrophomonas and Pseudomonas genera were also more abundant in an organic farming system20. However, contrasting results were also observed in a six-year-long organic cropping study27. Characterization of the specific microbes involved in soil nutrient cycling or soil disease suppression that are significantly impacted by organic farming is necessary. Such microbes may be used to as indicators to monitor soil health. Although much work has been carried out to evaluate the impact of organic farming practices on soil microbiota, many farms containing different crop species and in various climate zones should be analyzed to draw clear conclusions regarding the differences between organic and conventional farms25.
Cases studies showed that many factors were positively respond to the organic farming. Such as soil enzyme activity2,28, soil nutrients2,4, diversity of animals and plants9,10,11,12, and microbial abundance8. Meanwhile, all these indicators are directly or indirectly related to soil microorganisms. That raises a series of questions: Will microbial communities show similarly positive responses to organic farming under different crops plantation? What are the main microorganisms involved in responding to these positive changes? What ecological functions do these changed microorganisms possess? Organic farming developed rapidly in China and became disseminated around the country due to its food and environmental safety a. In this study, we cooperated with the Organic Food Development and Certification Center of China (OFDC), to investigate the relationship between the soil microbial community structure and organic farming. We selected 12 organic and conventional crop production systems, which are mainly distributed in central and eastern China, to explore the responses of the microbial community structure to organic farming.
Results
Soil physical and chemical indexes and soil enzyme activities
Among organic farming systems of different ages, compared with the conventional farms, the soil physical and chemical indexes of organic farms were significantly changed (Table S2). The ANOVA results (Table 1) show that (1) there were significant differences in pH (soil pH), OM (organic matter), TP (total P), VP (available P), NO3-N (soil nitrate nitrogen), NH4-N (ammonium nitrogen), TK (soil total K) and VK (available K) between conventional and organic farms (P < 0.01); However these soil physical-chemical factors were not significantly different among different crop types. There existed significantly (P < 0.01) interaction between production system and location, in pH, OM, TP, VP, NO3-N, NH4-N, TK and VK. Soil pH was slightly higher in the organic groups (Table S2). Even if different natural conditions exist in different regions, the organic group had higher (P < 0.05) content OM, TP, VP, TK and VK contents than the conventional group, with the exception of NO3-N, NH4-N and SBD (soil bulk density); These results showed that organic farms can maintain or even increase soil fertility more effectively, as evidenced by factors such as OM, TP, VP, TK and VK. More interestingly, we found no significant difference in TN (soil total N) between organic and conventional farms. However, the available nitrogen (NO3-N and NH4-N) in soils under organic management was significantly lower than that in soils under conventional management.
The enzyme activities in relation to the cycling of carbon (C), nitrogen (N) and phosphorus (P) were determined by the methods of Zhou et al. (Zhou73). Soil invertase, urease, and acid phosphatase activities in the OTW and OTC sites were significantly higher than in the corresponding CTW and CTC sites (Fig. 1). Soil invertase activity in the organic fields was significantly higher than that in the conventional field, but the soil urease enzyme activity was not significantly different between the OVL and CVL, OPJ and CPJ, OPS and CPS sites. Acid phosphatase enzyme activity in OVL, OTW and OTC was significantly higher than that in the corresponding conventional sites (CVL, CTW and CTC) (P < 0.05); However, this activity in the OPJ and OVY sites, which had a partial neutral pH, was lower compared with the conventional sites.
Richness and diversity of microbial communities
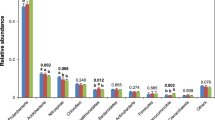
A total of 2,401,529 high-quality 16SV4–V5 sequences were obtained from all 36 samples were, with an average of 66,709 sequences (varying from 52,052 to 79,300) per sample (Table S3). A total of 10,865 OTUs were obtained, with 97% sequence similarity. Rarefaction curves showed that the paddy and vegetable soils had the steepest rarefaction curves with the highest taxon richness, whereas the tea soil had the lowest curves (Fig. S1). Organic farming had higher OTUs numbers than conventional farming, especially for tea soil.
Bacterial community richness was estimated with different richness estimators including Chao1 and the Abundance based Coverage Estimator (ACE). The results of Chao1 and ACE (Table 2) indicated that organic farming systems had greater microbial richness. Organic farming management had a significant positive effect on OVY, OTC, OTW and OPJ, whereas the vegetable soil of Lishui and the paddy soil of Shanghai were unaffected. The tea soils had lower richness (Chao 1 and ACE) and were more significantly affected by organic farming than the other two soils. According to Shannon and Simpson (Table 2), the organic group (except OPS) had a higher diversity, with the Shannon index ranging from 5.98 to 6.76, whereas the conventional group (except CPS) had a lower Shannon index, ranging from 4.91 to 6.11, and significant differences (P < 0.05) were evident between the two groups (Table 2). Similarly, the diversity in the tea, vegetable and paddy soils of Jurong was significantly higher under organic farming than under conventional farming, whereas no differences were found between organic OPS and conventional CPS.
Bacterial community structure
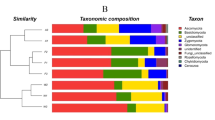
Principal component analysis (PCA) (Fig. 2A) was used to compare the similarity of the soil bacterial community among the samples. PCA was performed to compare organic and conventional management at the OTU level. PCA identified two principal component factors (PCF) in relation to the percentage abundance of groups, explaining 37.92% and 22.18% of the total variation. PCA showed that the two types of management could be separated and that the organic farming samples were inclined to cluster together with high similarity, whereas the conventional samples were more decentralized. These results suggested that the microbial community structures were significantly modified by organic farming. Under conventional management, the bacterial community had marked diversity among the different crop fields, whereas under organic management, the bacterial community had fewer differences among the 3 crops. Organic farming caused the soil bacterial community to be more consistent, although for a different type of crop. When crop type was considered individually, there was higher similarity among samples of paddy soil other than among samples of vegetable soil and tea soil, which indicates that vegetable soil and tea soil were more sensitive to the different management practices than paddy soil.
(A) PCA based on total OTUs level information, PC1 and PC2 were used to plot the result. The first letter of the field name represents the farming mode; “O” represents organic farming; “C” represents conventional farming; the second letter represents the crop type; “V” represents vegetable; “P” represents paddy; “T” represent tea; the third letter represents the experimental region (the first letter of the sampling area). (B) Similarity between organic and conventional samples. Euclidean distance between soil pairs is shown. (C) The Euclidean distance values of paddy, tea and vegetable soils. (D) The Euclidean distance values of paddy and upland soils. (E) The Euclidean distance values of S, J, L, Y, C and W. The nonparametric Wilcoxon test was used to calculate significance among the different sample groups (*P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant).
To compare community structure and similarity between organic and conventional samples, we determined the Euclidean distance between soil pairs. The Euclidean distance between organic pairs was significantly lower than that for conventional samples (Fig. 2B), supporting the observation that organic farming provided more similar bacterial community structure. At the same time, we also conducted the difference significance test on other kind of grouping (Fig. 2C–E). Different crops and water management practices (paddy soil and upland soils) exert influence on bacterial community. But organic or conventional management were still the important factor that influence the bacterial community. The shared OTUs for different crop types, locations and management models were determined via the Venn diagram (Fig. 3). For the vegetable and paddy soil, a total of 1490 OTUs (18.18%) could be detected in all four organic soils and 2607 OTUs (745 + 547 + 801 + 514; 31.76%) were unique to the soils they were found in (Fig. 3A1). In contrast, the shared OTUs were 609 (8.53%) only found in the conventional soils, and unique OTUs were 3501 OTUs (1056 + 779 + 1504 + 621; 41.32%) (Fig. 3A2). A similar pattern was observed for the vegetable and tea soil, in organic group, the number of shared and unique OTUs were 602 OTUs (7.90%) and 2921 OTUs (784 + 1108 + 525 + 504; 38.33%) (Fig. 3B1). But 463 OTUs (6.75%) and 3731 OTUs (2404 + 644 + 279 + 404; 54.38%) in conventional group (Fig. 3B2). Similar to all above results, for the paddy and tea soil, the percentage of shared and unique OTUs in the organic soils accounted for 316 and 3828 OTUs, respectively (Fig. 3C1). But 255 and 4739 OTUs in the pair of conventional (Fig. 3C2). All these result showed that the organic soil have more number and percent of shared OTUs, but lower number of unique OTUs.
For each crop type, we used redundancy analysis (RDA) to discern the OTU-level structure using the environmental variables (Fig. S2). The paddy, vegetable and tea soil samples were grouped according to the management system (O and C). The O and C management systems were differentiated along the first and second (or third for paddy soil) axes. These results suggest that pH, TP, TK and VK were key factors in shaping the microbial community functional structures in this system.
LEfSe analysis based on community abundance
Linear discriminate analysis (LDA) effect size (LEfSe) is an effective statistical tool for high-dimensional biomarker discovery and the explanation of the detailed identification of abundant features that characterize potential discriminating taxa between two or more biological groups29. LEfSe was used to evaluate the bacterial groups that were significantly different between the two management models. All 36 samples could be separated into organic and conventional groups. We chose LDA scores higher than 2 to identify bacterial groups with statistically significant differences (see Table S4 in the supplemental material).
The cladograms show taxa with LDA values higher than 2.5 for clarity (Fig. 4). Some major groups of bacteria were enriched in the organic group, namely, Flavobacteria. Actinomycetales (the order and family of Micromonosporaceae, Pseudonocardiaceae, and Streptosporangiaceae), Chthonomonadetes, Phycisphaerales, Spartobacteria, Erysipelotrichia, Rhizobiales (the order and families of Beijerinckiaceae, Hyphomicrobiaceae, Xanthobacteraceae, Rhizobiaceae, Phyllobacteriaceae and Bradyrhizobiaceae), Novosphingobium, Rhodospirillales, Myxococcales (the order and family of Phaselicystidaceae, Polyangiaceae, Nannocystineae, and Myxococcaceae), Desulfurellales, Thiotrichales, Alteromonadales, Cupriavidus. Within these groups, 5 fine lineages had an LDA value of 3 or higher, namely, Myxococcales, Planctomyces, Phycisphaera, Prosthecomicrobium and Lacibacter.
LEfSe cladogram of comparison result between organic and conventional samples.
The black circles from inner to outer stand for phylum, class, order, family, genus, and species. Green circles stand for taxa which were abundant in the organic group and red circles stand for taxa which were abundant in the conventional group.
The bacterial lineages enriched in the conventional soils were Proteobacteria, Gammaproteobacteria, Xanthomonadales, three groups in Actinomycetales (Microbacteriaceae, Dermacoccaceae and Nakamurellaceae), Ottowia, Ruminococcaceae, three groups in Clostridi (Sporobacter, Alicyclobacillu and Alkaliphilus), Ktedonobacteria, Sphaerobacterales, Sporolactobacillaceae, Acidiphilium, and Terriglobus. Only Gammaproteobacteria, Xanthomonadales, Proteobacteria and Xanthomonadaceae had LDA values higher than 4 in the conventional samples (Fig. 4).
Discussion
Organic farming has become one of the most popular sustainable strategies to produce agricultural products8. Compared with conventional farming, organic farming was purported to improve the soil ecosystem quality2,5. However, solid scientific evidence is needed to clarify the ecological effects of organic farming. Soil bacteria are important member of the soil ecosystem and indices of soil health30. Elucidation of the soil bacterial structure will shed light on the regulatory pattern of soil microbial population8,31.
Farm management directly affected the nutrient input2,4, and microbial community changed in response to the soil nutrients32. Organic management significantly raised the pH, contents of OM, TP, VP, VK and TK of the organic soils investigated in this research. Meanwhile, bacterial richness, bacterial diversity, and soil enzymes’ activity (invertase, urease, and acid phosphatase) also increased comparing to conventional farming. These results implied the linkage between soil management, soil nutrients and the soil microbial community. Microbial community fed back to soil nutrients by modifying soil enzyme activity2,28.
Our results showed that organic farming led to more richness and diversity than conventional farming. Recent studies show that rejuvenation of ecosystem function requires the restoration of species evenness, rather than richness alone33. Organic farming potentially offers a means of recovering functional evenness in an ecosystem33. A number of studies have confirmed that soil microbes are often more diverse and abundant under organic than conventional systems in various soils33,34,35,36. The abundance of some soil microbial groups was increased under an organic system.
PCA demonstrated that the two types of management could be separated at the OTU level (97% ID); the organic samples tended to cluster together with high similarity, whereas the conventional samples were more decentralized. Sequences sharing a 97% identity or higher are normally defined as a bacterial “species” although no rigorous species concept for bacteria exists37. The clustering together tendency was also observed in archived soils38. A comparative study of organic and conventional commercial olive farming systems, discriminant and NMDS analyses proved especially effective in differentiating the 93 olive orchards according to their management system (conventional, organic or wild olives)39. 14 olive orchards from Córdoba and Granada provinces in southern Spain could be distinguished according to their management (conventional, organic or integrated)40. These organic soils showed in general greater soil nutrients, enzyme activities and showed intermediate characteristics (such as pH and OM) which may be attributed to the fact that organic farming management led to a similar microbial community structure. In addition, there were similar agronomic management practices as those in organic farms (i.e., out of use of Pesticide, use of cover crops, animal grazing or light tillage to control weeds or incorporation of animal manure as fertilizer). Under organic farm, the organic manure incorporation may have fostered the bacterial pathway of the soil food web2. Organic amendments had a positive impact on soil microbiology, as suggested by the increased amount of microbial C, N and P41. The bacteria Co-correlation networks were strongly bimodal response to farmyard manure application42.
LEfSe was used to evaluate bacterial groups that showed a significant difference between the soils. In the present study, the results showed that different management practices had a significant effect on the soil microbial community. As shown in Fig. 4, the organic group evidenced multiple shifts of specific bacterial lineages distributed in a multitude of lineages, and most of these bacterial lineages were involved in nutrient cycling. For example, members of the genus Flavobacterium are responsible for heterotrophic denitrification43. Recent studies show that Verrucomicrobia are ubiquitous in soils and appear to be predominant in many soil bacterial communities44, which are predominantly comprised of the Class Spartobacteria44,45. Although we know very little about Verrucomicrobia, they are generally considered to be free-living disintegrators. The Sphingomonadaceae family has the ability to utilize a great diversity of C sources, including recalcitrant xenobiotic molecules46,47. Micromonosporaceae (a family within Actinomycetales) are associated with secondary metabolite production, and some species are efficient solubilizers of rock phosphate48. Some reports note that Micromonosporas can degrade complex recalcitrant materials such as cellulose and chitin49,50, and produce acid and alkaline phosphatases51. It is interesting to note that our results found multiple genera of Rhizobiales and Myxococcales. Rhizobiaceae (Sinorhizobium) is involved in N cycling52; Bradyrhizobiaceae (Rhodopseudomonas), Xanthobacteraceae (Starkeya) and the Hyphomicrobiaceae (Rhodoplanes) are mainly involved in C and N cycling. Under hypoxic conditions, the genera Bradyrhizobiaceae and Hyphomicrobiaceae can also utilize N2, NO3−, or NH353,54,55. Members of the Myxobacterial community act as micropredators that are metabolically active in the soil microbial food web56, and play a vital role in the turnover of carbon in soil ecosystems57,58. Alteromonadaceae is generally found in coastal environments, and have a potential activity in crude oil degradation59. The families Thiotrichaceae and Desulfurellaceae are mainly involved in S cycling60. In this study, invertase, urease, and acid phosphatase activities increased under organic farming, indicating that a changes in bacterial communities increased soil nutrient cycling.
In comparison, the relative abundances of Gammaproteobacteria, Xanthomonadales, Ruminococcaceae, Sphaerobacterales, Acidiphilium, Acidobacteriaceae, Sporolactobacillaceae, Alicyclobacillu and Alkaliphilus were significantly lower in organic than in conventional soil. The genus Xanthomonadales has anti-microbial activity against competing rhizosphere microorganisms30. A greater abundance of acid and alkali resistant microorganisms (Acidiphilium, Acidobacteriaceae, Sporolactobacillaceae, Alicyclobacillu and Alkaliphilus) were found under conventional tillage, suggesting that conventional tillage may exert stress on soil microbiota and that organic tillage is an eco-friendly pattern that encourages sustainable development.
A shift in particular bacterial lineages caused by organic management could be used as a bioindicator to assess soil quality in an agricultural production system, or used for the production of bio-organic fertilizer. For example, B. asahii originated from an archived OM fertilized soil sample61, which played a key role in increases in both crop yield and soil fertility, especially by accelerating carbon and phosphorus cycling.
Organic management of soil is generally considered to enhance soil disease suppression. This phenomenon has been found in many crops, such as tomato62, wheat63, barley64,65 and apple trees66. Various soil physicochemical factors and some disease-suppressing bacterial taxa can contribute to disease suppression30,67. Disease- suppressive bacteria were reported to play key roles in soil suppression. Firmicutes, and Actinobacteria, which were enriched in organic farming, were consistently associated with disease suppression30. In addition, certain bacterial genera, such as Pseudomonas, Bacillus, Burkholderia, and Actinomycetes, were often found in high populations in soils that were disease suppressive67. It is interesting that Myxococcales (Cystobacteraceae and Haliangiaceae), which were enriched on organic farms, have the capacity to produce secondary metabolites that have antibacterial and antifungal activities, such as Althiomycin and Myxovirescin68,69,70. A previous work showed that myxobacteria effectively kill microbial pathogens71, and exert partial control of damping-off and root disease in container-grown tree seedlings72. Populations of myxobacteria were stable. Zhou56 retrieved 103 pyrosequencing datasets in various soil samples from the MG-RAST and SRA databases to determine the abundance of myxobacteria. Their result showed that myxobacteria were among the predominant bacterial groups, accounting for 0.40–4.50% of the bacterial sequences. In our 36 soils, myxobacteria accounted for 0.25–4.02% of the bacterial sequences. We suspected that myxobacteria was a potential disease-suppressing bacteria, and played a key role in organic farming. However, further research is needed.
Miseq sequencing technique was used to analyze the consistent responses of bacterial community to organic farming for its ability of providing huge amount of microbial information. Our results showed that organic farming tends to maintain a highly similar microbial community structures regardless of crop types planted or soil management practices and soil types. Organic farming leads to a more stable microflora, and is beneficial to soil health and sustainable use of soil. Organic agriculture significantly increased the abundance of nutrition-related bacteria such as Rhizobiales, Thiotrichaceae, Micromonosporaceae, Desulfurellaceae and Myxococcales, and reduced the abundance of acid and alkali resistant bacteria.
In conclusion, organic farming significantly improved soil nutrient status and soil enzyme activity and enhanced the richness and diversity of the soil bacterial community. The microbial community in organic soils tended to converge at the OTU level. Dominant guilds involved in nutrient (C, N, S and P) cycling were significantly enriched, leading to new dynamics between the C, N, P and S cycles. Soil pH and soil nutrients were found to be nearly equally important in influence the microbial community, and the composition of the soil microbial community was strongly correlated with soil nutrients such as TP, VP, NH4-N, TK and VK in both organic and conventional systems. Organic farming promoted the uniformity of the bacterial community structure for all type of crops investigated. Compared with rice and vegetable crop soils from east China, tea soils from central China were more responsive to organic farming. All of these results allow us to better understand the differences between organic and conventional farming for different crop systems and the linkage between soil management, soil nutrients and the soil microbial community.
Materials and Methods
Study area
Rice, vegetable and tea are the most common crops in eastern and central China and are widely grown in this region for the conventional and organic markets. We relied on the OFDC to investigate the effects of organic management on microbial communities in agricultural soil and used conventional farming as a control. We finally selected 6 organic crop production systems (Certified organic by OFDC) and 6 corresponding conventional crop production systems. The vegetable soils were collected at Lishui [labeled as OVL (119°02′E, 31°39′N) and CVL (118°58′E, 31°36′N)], and Yangzhou [labeled as OVY (119°8′E, 32°25′N) and CVY (119°8′E, 32°22′N)] in Jiangsu Province, in China. The paddy soils were collected at Shanghai [labeled as OPS (121°12′E, 31°48′N) and CPS (121°12′E, 31°45′N)], and Jurong [labeled as OPJ (119°13′E, 31°39′N) and CPJ (119°13′E, 31°41′N)], in Jiangsu Province, in China. The tea soils were collected at Wuyuan [labeled as OTW (117°44′E, 29°26′N) and CTW (117°49′E, 29°20′N)], in Jiangxi Province and from Changsha [labeled as OTC (113°19′E, 28°33′N) and CTC (113°20′E, 28°34′N)], in Hunan Province, in China. Nutrient input and weed control varied among farms (Table S1), but these actual operational farms more accurately reflected actual microbial community information.
Soil sampling and analysis
The sampling sites are shown in Table S1. The sampling spatial coordinates were recorded by GPS. Surface soil samples were collected in September 2013. In each field, three plots were established at random locations within a 0.25 ha area (50 m × 50 m). Soil samples were collected from each plot at 12 points at a depth of 0-20 cm, situated 15 cm from the centerline of the planting row, and then mixed and homogenized by passing through a < 2 mm sieve to remove aboveground roots, visible residue, and stones. The fresh soil samples were stored in polyethylene bags and subdivided into two subsamples; One was stored at 4 °C to determine its physical and chemical properties, and the other was stored at −20 °C prior to DNA extraction and microbial analysis.
Soil invertase, urease, and acid phosphatase activities were measured using the method of Zhou et al.73. Soil pH was determined with a glass electrode as previously described74. Soil total N (TN) and organic matter (OM) were determined by Kjeldahl digestion75 and determined by oil bath–K2CrO7 titration method76. Soil total K (TK) and total P (TP) were determined by digestion with HF-HClO477, followed by flame photometry and molybdenum-blue colorimetry78, respectively. Available K (VK) was extracted by ammonium acetate and determined by flame photometry79. Available P (VP) was extracted by sodium bicarbonate and determined using the molybdenum blue method80. Soil nitrate nitrogen (NO3–N) and ammonium nitrogen (NH4–N) were extracted from 15 g of fresh soil with 2 M KCl (soil: extract/1:5) and analyzed using a continuous flow analytical system (San++ System, Skalar, Holland)81.
DNA extraction and PCR amplification
DNA was extracted from the soil samples using an Ultra Clean microbial DNA isolation kit and from soil samples using the FastDNA® SPIN Kit for soil (MP Biomedicals, Santa Ana, CA) following the manufacturer’s instructions. All samples were crushed using a FastPrepTM FP120 machine (MP Biomedicals) using Lysing Matrix A tubes at speed level 4, three times for 20 s each. The primers 515F (5′-CCTACGGGAGGCAGCAG-3′) and 907R (5′-TTACCGCGGCTGCTGGC-3′) were chosen to amplify the V4–V5 hypervariable region of the 16S rRNA gene. The 20 μl PCR reaction mix consisted of 10 ng of template DNA,4 μl of 5×FastPfu Buffer, 2 μl of dNTP mix (2.5 mM each), 0.4 μl of Fast Pfu Polymerase (2.5 units), 0.4 μl of 10 μM barcode primer 515F, 0.4 μl of 10 μM primer 907R, and 12.75 μl of double distilled water. After denaturing at 94 °C for 2 min, the amplification was carried out with 25 cycles of 30 s at 94 °C, 30 s at 55 °C, 45 s at 72 °C and a final extension step at 72 °C for 10 min. The PCR products were purified with the PCR Clean-UpTM kit (MO BIO Labs, Solana Beach, CA, USA) and used as a template for direct sequencing.
Sequencing using MiSeq PE300
Amplicons were sent out for pyrosequencing on the Illumina platform. The Majorbio-Shanghai. Illumina MiSeq PE300 was used to sequence the samples. After sequencing, the Trimmomatic82 was used to process the raw sequences, and the PE reads were overlapped by flash83 to assemble the final V4-V5 tag sequences. The uparse method (version 7.1) on the Usearch software platform (version 7.1 http://qiime.org/)84 was employed to assign operational taxonomic units (OTUs) to the 16S rRNA at a cutoff level of 3%. The Uchime (version 4.2.40)85 method was used to correctly detect and remove chimeras produced by PCR. To compare relative differences between the samples, a randomly selected subset of 41105 sequences per sample was performed for downstream analyses.
Statistical analysis
An analysis of variance (ANOVA) was performed to evaluate the main effects of the agricultural system, farm location, crop type and the interactions of these factors on the soil physical and chemical analyzed. Based on the OTU results, the rarefaction curve and Shannon index curve were analyzed using Mothur (version v.1.30.1)86, and the richness estimators (Chao1, Abundance-based and Coverage Estimator (ACE)) and diversity indices (Shannon and Simpson) were determined using Mothur. Principal component analysis (PCA) and redundancy analysis (RDA) was performed with the rda() function in the vegan package in R (Version 3.0.2, vegan package)87. A Venn diagram was generated using R (Version 3.0.2, venn Diagram package). LEfSe29 was used to find indicator bacterial groups specialized within the organic and conventional types of samples. Statistical analysis was conducted using SPSS 13.0.
Additional Information
How to cite this article: Wang, W. et al. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze River. Sci. Rep. 6, 35046; doi: 10.1038/srep35046 (2016).
References
Tilman, D. et al. Forecasting agriculturally driven global environmental change. Science 292, 281–284 (2001).
Mäder, P. et al. Soil fertility and biodiversity in organic farming. Science 296, 1694–1697 (2002).
Pelletier, N., Arsenault, N. & Tyedmers, P. Scenario modeling potential eco-efficiency gains from a transition to organic agriculture: Life cycle perspectives on Canadian canola, corn, soy, and wheat production. Environmental management 42, 989–1001 (2008).
Syswerda, S., Basso, B., Hamilton, S., Tausig, J. & Robertson, G. Long-term nitrate loss along an agricultural intensity gradient in the Upper Midwest USA. Agriculture, Ecosystems & Environment 149, 10–19 (2012).
Cavigelli, M. A., Mirsky, S. B., Teasdale, J. R., Spargo, J. T. & Doran, J. Organic grain cropping systems to enhance ecosystem services. Renewable agriculture and food systems 28, 145–159 (2013).
Drinkwater, L., Letourneau, D., Workneh, F., Van Bruggen, A. & Shennan, C. Fundamental differences between conventional and organic tomato agroecosystems in California. Ecological Applications 1098–1112 (1995).
Stamatiadis, S., Werner, M. & Buchanan, M. Field assessment of soil quality as affected by compost and fertilizer application in a broccoli field (San Benito County, California). Applied Soil Ecology 12, 217–225 (1999).
Smith, J. et al. Organic Farming and Biodiversity: A review of the literature. Organic Center Wales, Aberystwyth: Wales (2011).
Fuller, R. et al. Benefits of organic farming to biodiversity vary among taxa. Biology letters 1, 431–434 (2005).
Gabriel, D. et al. Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecology letters 13, 858–869 (2010).
Feber, R., Johnson, P., Firbank, L., Hopkins, A. & Macdonald, D. A comparison of butterfly populations on organically and conventionally managed farmland. Journal of Zoology 273, 30–39 (2007).
Schmidt, M. H., Roschewitz, I., Thies, C. & Tscharntke, T. Differential effects of landscape and management on diversity and density of ground‐dwelling farmland spiders. Journal of Applied Ecology 42, 281–287 (2005).
Gregorich, E., Monreal, C., Carter, M., Angers, D. & Ellert, B. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Canadian journal of soil science 74, 367–385 (1994).
Seklemova, E., Pavlova, A. & Kovacheva, K. Biostimulation-based bioremediation of diesel fuel: field demonstration. Biodegradation 12, 311–316 (2001).
Young, I. M. & Crawford, J. W. Interactions and self-organization in the soil-microbe complex. Science 304, 1634–1637 (2004).
Bünemann, E., Schwenke, G. & Van Zwieten, L. Impact of agricultural inputs on soil organisms—a review. Soil Research 44, 379–406 (2006).
Nelson, A. G. & Spaner, D. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming 217–242 (Springer, 2010).
Esperschütz, J., Gattinger, A., Mäder, P., Schloter, M. & Fließbach, A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiology Ecology 61, 26–37 (2007).
Hartmann, M., Frey, B., Mayer, J., Mäder, P. & Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. The ISME journal (2014).
Li, R. et al. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities (2012).
Gattinger, A. et al. Enhanced top soil carbon stocks under organic farming. Proceedings of the National Academy of Sciences 109, 18226–18231 (2012).
He, J.-Z., Ge, Y., Xu, Z. & Chen, C. Linking soil bacterial diversity to ecosystem multifunctionality using backward-elimination boosted trees analysis. Journal of Soils and Sediments 9, 547–554 (2009).
Wittebolle, L. et al. Initial community evenness favours functionality under selective stress. Nature 458, 623–626 (2009).
Van Bruggen, A. H. Plant disease severity in high-input compared to reduced-input and organic farming systems. Plant disease 79, 976–984 (1995).
Sugiyama, A., Vivanco, J. M., Jayanty, S. S. & Manter, D. K. Pyrosequencing assessment of soil microbial communities in organic and conventional potato farms. Plant Disease 94, 1329–1335 (2010).
Schmid, F., Moser, G., Müller, H. & Berg, G. Functional and structural microbial diversity in organic and conventional viticulture: organic farming benefits natural biocontrol agents. Applied and environmental microbiology 77, 2188–2191 (2011).
Grantina, L. et al. Impact of six-year-long organic cropping on soil microorganisms and crop disease suppressiveness. Zemdirbyste- Agriculture 98, 399–408 (2011).
García-Ruiz, R., Ochoa, V., Hinojosa, M. B. & Carreira, J. A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biology and Biochemistry 40, 2137–2145 (2008).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol 12, R60 (2011).
Mendes, R. et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100 (2011).
Janvier, C. et al. Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biology and Biochemistry 39, 1–23 (2007).
Yu, C. et al. Changes in soil microbial community structure and functional diversity in the rhizosphere surrounding mulberry subjected to long-term fertilization. Applied Soil Ecology 86, 30–40 (2015).
Crowder, D. W., Northfield, T. D., Strand, M. R. & Snyder, W. E. Organic agriculture promotes evenness and natural pest control. Nature 466, 109–112 (2010).
Klaus, V. H. et al. Does organic grassland farming benefit plant and arthropod diversity at the expense of yield and soil fertility? Agriculture, Ecosystems & Environment 177, 1–9 (2013).
Hole, D. et al. Does organic farming benefit biodiversity? Biological conservation 122, 113–130 (2005).
Whittingham, M. J. The future of agri‐environment schemes: biodiversity gains and ecosystem service delivery? Journal of applied ecology 48, 509–513 (2011).
Goodrich, J. K. et al. Conducting a microbiome study. Cell 158, 250–262 (2014).
Dolfing, J. et al. Microbial diversity in archived soils. Science (New York, NY) 306, 813 (2004).
Montes-Borrego, M., Navas-Cortés, J. A. & Landa, B. B. Linking microbial functional diversity of olive rhizosphere soil to management systems in commercial orchards in southern Spain. Agriculture, ecosystems & environment 181, 169–178 (2013).
Benitez, E., Nogales, R., Campos, M. & Ruano, F. Biochemical variability of olive-orchard soils under different management systems. Applied Soil Ecology 32, 221–231 (2006).
Blanchet, G., Gavazov, K., Bragazza, L. & Sinaj, S. Responses of soil properties and crop yields to different inorganic and organic amendments in a Swiss conventional farming system. Agriculture, Ecosystems & Environment 230, 116–126 (2016).
Hartmann, M., Frey, B., Mayer, J., Mader, P. & Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. The ISME Journal 9 (2015).
Payne, W. J. Denitrification. (John Wiley & Sons Inc., 1981).
Bergmann, G. T. et al. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biology and Biochemistry 43, 1450–1455 (2011).
Eilers, K. G., Debenport, S., Anderson, S. & Fierer, N. Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biology and Biochemistry 50, 58–65 (2012).
Adkins, A. Degradation of the phenoxy acid herbicide diclofop-methyl by Sphingomonas paucimobilis isolated from a Canadian prairie soil. Journal of Industrial Microbiology and Biotechnology 23, 332–335 (1999).
Pinyakong, O., Habe, H. & Omori, T. The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons (PAHs). The Journal of general and applied microbiology 49, 1–19 (2003).
Hamdali, H., Hafidi, M., Virolle, M. J. & Ouhdouch, Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. applied soil ecology 40, 510–517 (2008).
Talia, P. et al. Biodiversity characterization of cellulolytic bacteria present on native Chaco soil by comparison of ribosomal RNA genes. Research in microbiology 163, 221–232 (2012).
Jendrossek, D., Tomasi, G. & Kroppenstedt, R. M. Bacterial degradation of natural rubber: a privilege of actinomycetes? FEMS Microbiology letters 150, 179–188 (1997).
El-Tarabily, K. A., Nassar, A. H. & Sivasithamparam, K. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Applied soil ecology 39, 161–171 (2008).
Prescott, L., Harley, J. & Klein, D. Clinical microbiology. Microbiology 725 (2005).
Larimer, F. W. et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nature biotechnology 22, 55–61 (2003).
Kulichevskaya, I. S., Guzev, V. S., Gorlenko, V. M., Liesack, W. & Dedysh, S. N. Rhodoblastus sphagnicola sp. nov., a novel acidophilic purple non-sulfur bacterium from Sphagnum peat bog. International journal of systematic and evolutionary microbiology 56, 1397–1402 (2006).
Hiraishi, A. & Imhoff, J. Rhodoplanes Hiraishi and Ueda 1994b, 671 VP. Bergey’s Manual® of Systematic Bacteriology. 545–549 (2005).
Zhou, X. w. et al. Myxobacterial community is a predominant and highly diverse bacterial group in soil niches. Environmental microbiology reports 6, 45–56 (2014).
Lueders, T., Kindler, R., Miltner, A., Friedrich, M. W. & Kaestner, M. Identification of bacterial micropredators distinctively active in a soil microbial food web. Applied and Environmental Microbiology 72, 5342–5348 (2006).
Reichenbach, H. The ecology of the myxobacteria. Environmental microbiology 1, 15–21 (1999).
Patel, V., Munot, H., Shouche, Y. S. & Madamwar, D. Response of bacterial community structure to seasonal fluctuation and anthropogenic pollution on coastal water of Alang–Sosiya ship breaking yard, Bhavnagar, India. Bioresource technology 161, 362–370 (2014).
Park, S. et al. Microbial community structure and dynamics in a mixotrophic nitrogen removal process using recycled spent caustic under different loading conditions. Bioresource technology 102, 7265–7271 (2011).
Feng, Y. et al. Bacillus asahii comes to the fore in organic manure fertilized alkaline soils. Soil Biology and Biochemistry 81, 186–194 (2015).
Grünwald, N., Hu, S. & Van Bruggen, A. Short-term cover crop decomposition in organic and conventional soils: characterization of soil C, N, microbial and plant pathogen dynamics. European Journal of Plant Pathology 106, 37–50 (2000).
Hiddink, G. A., van Bruggen, A. H., Termorshuizen, A. J., Raaijmakers, J. M. & Semenov, A. V. Effect of organic management of soils on suppressiveness to Gaeumannomyces graminis var. tritici and its antagonist, Pseudomonas fluorescens. European Journal of Plant Pathology 113, 417–435 (2005).
Knudsen, I. M., Debosz, K., Hockenhull, J., Jensen, D. F. & Elmholt, S. Suppressiveness of organically and conventionally managed soils towards brown foot rot of barley. Applied Soil Ecology 12, 61–72 (1999).
Rasmussen, P. H., Knudsen, I. M., Elmholt, S. & Jensen, D. F. Relationship between soil cellulolytic activity and suppression of seedling blight of barley in arable soils. Applied Soil Ecology 19, 91–96 (2002).
Manici, L., Ciavatta, C., Kelderer, M. & Erschbaumer, G. Replant problems in South Tyrol: role of fungal pathogens and microbial population in conventional and organic apple orchards. Plant and Soil 256, 315–324 (2003).
van Bniggen, A. H. & Termorskuizen, A. J. Integrated approaches to root disease management in organic farming systems. Australasian Plant Pathology 32, 141–156 (2003).
Proksch, P., Edrada-Ebel, R. & Ebel, R. Drugs from the sea-opportunities and obstacles. Marine Drugs 1, 5–17 (2003).
Weissman, K. J. & Müller, R. A brief tour of myxobacterial secondary metabolism. Bioorganic & medicinal chemistry 17, 2121–2136 (2009).
Gaspari, F. et al. Myxobacteria isolated in Israel as potential source of new anti‐infectives. Journal of applied microbiology 98, 429–439 (2005).
Bull, C., Shetty, K. & Subbarao, K. Interactions between myxobacteria, plant pathogenic fungi, and biocontrol agents. Plant Disease 86, 889–896 (2002).
Hocking, D. & Cook, F. D. Myxobacteria exert partial control of damping-off and root disease in container-grown tree seedlings. Canadian journal of microbiology 18, 1557–1560 (1972).
Zhou, L. Soil enzymology. Science and Technology Press, Beijing, China (1987).
Feng, Y. et al. pH is a good predictor of the distribution of anoxygenic purple phototrophic bacteria in Arctic soils. Soil Biology and Biochemistry 74, 193–200 (2014).
Bremner, J. & Mulvaney, C. Methods of soil analysis, Part 2. American Society of Agronomy, 1162–1164 (1965).
Kong, X., Zhang, F., Wei, Q., Xu, Y. & Hui, J. Influence of land use change on soil nutrients in an intensive agricultural region of North China. Soil and Tillage Research 88, 85–94 (2006).
Jacson, M. Soil chemical analysis. Soil chemical analysis (1958).
Chu, H. et al. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biology and Biochemistry 39, 2971–2976 (2007).
Carson, P. L. Recommended potassium test. Bull Dep Agric Econ ND Agric Exp Stn ND State Univ Agric Appl Sci (1975).
Olsen, S. R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate (1954).
Sun, R., Zhang, X.-X., Guo, X., Wang, D. & Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biology and Biochemistry (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, btu170 (2014).
Magoč, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963 (2011).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature methods 10, 996–998 (2013).
Schloss, P. D., Gevers, D. & Westcott, S. L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PloS one 6, e27310 (2011).
Oksanen, J. et al. The vegan package. Community ecology package (2007).
Acknowledgements
This work was financially supported by the National Key R&D Program (2016YFD0200309), the “973” program (2015CB150502), the National Natural Science Foundation of China (No. 41371319), the Special Funds for Enviro-scientific Research (No. 2013467036), the Science and technology support program of Jiangsu province (No. BE2014741), and Genetically Modified Organisms Breeding Major Projects of China (Grant No. 2014ZX08011-003). We thank Biomics (Beijing) Biotech Co. Ltd for assistance in bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
H.W., X.X., X.Y. and Z.C. initiated and designed the research, W.W., X.L., X.Y. and Y.H. performed the experiments, W.W., Y.F. and L.W. analyzed the data and wrote the paper, R.S. and Z.Z. also revised and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, W., Wang, H., Feng, Y. et al. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze River. Sci Rep 6, 35046 (2016). https://doi.org/10.1038/srep35046
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35046
This article is cited by
-
A framework for the targeted recruitment of crop-beneficial soil taxa based on network analysis of metagenomics data
Microbiome (2023)
-
Evaluation, quantification, and mapping of ecosystem services in canola agroecosystems
Landscape and Ecological Engineering (2023)
-
Sugarcane cultivation practices modulate rhizosphere microbial community composition and structure
Scientific Reports (2022)
-
Different soil salinity imparts clear alteration in rhizospheric bacterial community dynamics in rice and peanut
Archives of Microbiology (2022)
-
Influence of Rosaceous Species and Driving Factors on Differentiation of Rhizospheric Bacteria in a Deciduous Broad-Leaved Forest
Current Microbiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.