Abstract
The actin cytoskeleton is a critical regulator of intestinal mucosal barrier permeability, and the integrity of epithelial adherens junctions (AJ) and tight junctions (TJ). Non muscle myosin II (NM II) is a key cytoskeletal motor that controls actin filament architecture and dynamics. While NM II has been implicated in the regulation of epithelial junctions in vitro, little is known about its roles in the intestinal mucosa in vivo. In this study, we generated a mouse model with an intestinal epithelial-specific knockout of NM IIA heavy chain (NM IIA cKO) and examined the structure and function of normal gut barrier, and the development of experimental colitis in these animals. Unchallenged NM IIA cKO mice showed increased intestinal permeability and altered expression/localization of several AJ/TJ proteins. They did not develop spontaneous colitis, but demonstrated signs of a low-scale mucosal inflammation manifested by prolapses, lymphoid aggregates, increased cytokine expression, and neutrophil infiltration in the gut. NM IIA cKO animals were characterized by a more severe disruption of the gut barrier and exaggerated mucosal injury during experimentally-induced colitis. Our study provides the first evidence that NM IIA plays important roles in establishing normal intestinal barrier, and protection from mucosal inflammation in vivo.
Similar content being viewed by others
Introduction
Establishment of the intestinal epithelial barrier is a fundamental feature of healthy gut, protecting the body from the harmful contents of the gut lumen, and allowing for the regulated bidirectional transport of fluids, nutrients, and waste. Disruption of the gut barrier plays an important role in the pathogenesis of different immune disorders, such as inflammatory bowel disease (IBD), celiac disease, sepsis, and diabetes1,2. The permeability of the intestinal epithelial barrier is regulated by specialized adhesive structures known as tight junctions (TJ) and adherens junctions (AJ). AJ are responsible for initiating cell-cell contacts3,4,5, whereas TJ create the seal between adjacent epithelial cells2,6,7,8. These junctional complexes are composed of adhesive transmembrane proteins and, associated with them, cytosolic plaque proteins. The transmembrane proteins of epithelial TJ include members of the claudin family, occludin, and junctional adhesion molecule-A (JAM-A)2,6,7,8, whereas E-cadherin and nectins comprise the major adhesive components of AJ3,4,5. Cytosolic plaques of AJ and TJ contain a variety of scaffolding, signaling, and trafficking proteins including α-catenin, β-catenin, p120-catenin, and members of the zonula occludens (ZO) protein family2,3,4,6,9,10.
Both AJ and TJ are physically associated with the prominent cortical actin cytoskeleton11,12. This association is critical for the assembly of epithelial junctions and the establishment of a paracellular barrier. Furthermore, remodeling of the actin cytoskeleton provides the force that drives TJ and AJ disassembly induced by environmental stressors and proinflammatory agents11,12,13. The integrity of the perijunctional actin cytoskeleton is regulated by a large number of motor, actin binding, and signaling proteins. The roles of these cytoskeletal regulators in the function of the normal intestinal epithelial barrier, as well as in barrier dysfunctions in inflamed gut, remain poorly understood.
Perijunctional actin filaments are enriched in non muscle myosin II (NM II), a motor protein that converts the chemical energy of ATP hydrolysis into mechanical forces, thus mediating cytoskeletal tension and contractility. This protein works as a molecular ensemble consisting of two heavy chains, two essential, and two regulatory myosin light chains (RMLC)14,15. NM II heavy chains comprise the major structural component of this cytoskeletal motor. Each heavy chain has a globular head, which binds to actin filaments and hydrolyzes ATP, and an extended tail that coils together with another heavy chain tail to form rigid rod-like myosin filaments14,15. Such high-order organization of NM II is critical for the cross-linking and movement of actin filaments. Phosphorylation of RMLC by myosin light chain kinase (MLCK) or Rho kinase (ROCK) is known to alter the conformation of heavy chains, thereby increasing NM II activity14,15. A number of previous studies implicated NM II heavy chain activity and RMLC phosphorylation in controlling all the steps of junctional dynamics (assembly, maintenance, and disassembly) in cultured intestinal epithelial cell monolayers in vitro16,17,18,19,20,21,22,23. However, little is known regarding how NM II motor regulates the gut barrier in vivo. A well-established concept in the field postulates that NM II activation triggered by MLCK-dependent phosphorylation of RMLC, disrupts the epithelial barrier during intestinal inflammation1,2,10,24. This concept is based on studies demonstrating that either genetic or pharmacological alterations to MLCK activity and RMLC phosphorylation affect the integrity of the intestinal barrier and the severity of experimental colitis in some17,25,26, but not all animal models25,27. However, these results should be considered with caution. Despite the common belief that RMLC uniquely associates with NM II, previous reports illustrate promiscuous binding of RMLC to other proteins that include the heavy chains of unconventional myosins from classes 14,15, 18, and 1928, as well as non-myosin targets, such as a bile acid transporter29, calponin30, etc. Moreover, evidence exist that MLCK can modulate junctional permeability in inflamed tissue via NM II-independent mechanisms involving integrin signaling31.
These examples emphasize the need for more specific experimental approaches to examine the effects of NM II activity on the integrity of epithelial barriers in vivo. The best approach would involve targeting NM II heavy chains considering their direct and indispensable roles in actin filament assembly and dynamics. It is noteworthy that regulation of NM II activity is not limited to RMLC phosphorylation, but also involves posttranslational modifications of the heavy chains as well as their interactions with different scaffolding proteins14,15,32. Epithelial cells express three different NM II heavy chain isoforms, A, B, and C, each with distinct enzymatic properties and cellular functions14,15,32,33. Our previous studies have demonstrated that NM IIA plays unique roles in regulating the paracellular barrier, and AJ/TJ remodeling in intestinal and pancreatic epithelial cell monolayers in vitro18,34. Furthermore, mislocalization of epithelial NM IIA was observed in the inflamed colonic mucosa of Crohn’s disease patients35. However, the functions of NM II heavy chains (motors) in regulating epithelial barrier in healthy gut, and during mucosal inflammation, remain unexplored. This study demonstrates, for the first time, that intestinal epithelial NM IIA controls the integrity of mucosal barrier in healthy gut in vivo, and limits the development of experimental colitis.
Results
Characterization of conditional knockout of NM IIA in the intestinal epithelium
Total knockout of NM IIA in mice is embryonically lethal36. In order to investigate the functions of this motor protein in the gut, we generated mice with intestinal epithelium-specific knockout of NM IIA by crossing NM IIA floxed animals with villin-Cre mice. The efficiency and specificity of NM IIA knockout was examined by immunoblotting analysis of intestinal epithelial cell scrapes and tissue samples collected from different organs. Intestinal scraping is a simple and convenient technique to collect tissue fractions enriched in epithelial cell markers and depleted of mesenchymal/smooth muscle cell markers (Supplementary Figure 1A). Immunoblotting analysis confirmed the selective loss of NM IIA expression in colonic and ileal epithelium without significant changes to its expression in the brain, kidney, lungs, and liver (Fig. 1A, Supplementary Figure 1). This knockout was specific for NM IIA and did not affect the expression of closely-related NM IIB and NM IIC isoforms (Fig. 1A). NM IIA flox/villin Cre homozygous animals (abbreviated hereafter as NM IIA cKO) appeared to be healthy. They gained body weight similar to control littermates and did not show spontaneous diarrhea or rectal bleeding (data not shown). The only phenotypic abnormality of NM IIA cKO mice was the development of rectal prolapses that were observed in approximately 52% of NM II cKO mice, but not in NM IIA+/+ or heterozygous animals (Fig. 1B, Table 1). Similar rectal prolapses were previously reported in different murine models of colitis, including interleukine-10 knockout mice and mice with the Th1 mucosal immune response to trinitrobenzoic acid37,38,39,40. The development of rectal prolapses is considered a sign of mucosal inflammation, although this phenotype is not an obligate consequence of inflammation, and was observed only in a fraction (8–67%) of the animals with colitis37,38,39,40.
(A) Immunoblotting analysis of the expression of different NM II isoforms in colonic epithelial scrapes obtained from NM IIA+/+ and NM IIA cKO mice. (B) Spontaneous development of rectal prolapse in NM IIA cKO animals (arrow). (C) Normal architecture of colonic epithelium and the formation of large lymphoid aggregates (arrow) in the distal colon of NM IIA cKO mice. (D) Periodic acid-Shiff-Alcian Blue staining of Goblet cells in the colonic mucosa of control and NM IIA cKO animals. Numbers in parentheses indicate the number of animals in each experimental group. Data is presented as mean ± SE; *P < 0.01. Scale bar, 50 μm.
Histological analysis reveals that intestinal epithelium-specific loss of NM IIA did not induce gross morphological alterations of normal colonic mucosa (Fig. 1C, middle image). However, approximately 42% of NM IIA cKO mice developed large lymphoid aggregates in the distal colon (Fig. 1C, arrow, and Table 1). Such lymphoid aggregates, along with the described rectal prolapses, could be indicative of low-scale mucosal inflammation in NM IIA cKO animals40,41. Another effect of intestinal epithelial NM IIA knockout was a dramatic decrease in the number of mucin granules in colonic Goblet cells, as indicated by Periodic Acid-Shiff and Alcian Blue staining (Fig. 1D). Such depletion of mucin granules was not accompanied by decreased mRNA expression of mucin-2 or molecular components of the Notch signaling pathway (Notch1, Hes1, and Math1), which plays a key role in Goblet cell differentiation (Supplementary Figure 2A). Therefore, the loss of mucin granules in NM IIA cKO colon is unlikely to reflect abnormal Goblet cell differentiation, but rather could be caused by either defective granule biogenesis or accelerated granule release due to mild mucosal inflammation. Moreover, the expression of Paneth cell markers, lysozyme and defensins, and the morphology of Paneth cell granules were not affected by the conditional loss of NM IIA in small intestine (Supplementary Figure 2B,C).
Intestinal specific knockout of NM IIA disrupted normal gut barrier in vivo
Considering the previous reports that inhibition of the NM II motor induces permeability defects in model intestinal epithelial monolayers18,20 we sought to investigate the effects of NM IIA knockdown on the integrity of gut barrier in vivo. Remarkably, unchallenged NM IIA cKO mice demonstrated approximately 6-fold increase in the transmucosal flux of FITC-dextran as compared to NM IIA+/+ animals, thereby indicating a leaky gut barrier (Fig. 2A). Such barrier disruption was accompanied by a selective decrease in the expression of two AJ cytoplasmic plaque proteins, β-catenin and p120 catenin (Fig. 2B), whereas expression of E-cadherin and all other major TJ proteins remained unchanged. Immunofluorescence labeling revealed a loss in apical junctional staining of β-catenin and p120 catenin in colonic crypts of NM IIA cKO mice (Fig. 2C,D arrows). Furthermore, a marked redistribution of claudin-7 from the intercellular junctions into cytosolic compartments, was observed in the colonic mucosa of NM IIA cKO animals (Fig. 3, arrowheads). In contrast, junctional localization of E-cadherin, occludin, and ZO-1, was not changed (Fig. 3; Supplementary Figure 3). Immunofluorescence labeling demonstrated enrichment of NM IIA at the apical and lateral plasma membrane in the colonic epithelium of NM IIA+/+ mice (Fig. 4A arrows), and loss of this labeling in NM IIA cKO animals. Interestingly, the loss of NM IIA did not affect the assembly of perijunctional F-actin bundles in either colonic crypt or surface epithelium (Fig. 4B arrowheads). Immunofluorescence analysis of the localization of other NM II isoforms revealed enrichment of NM IIC at the apical pole of NM IIA+/+ enterocytes, while NM IIB labeling was limited to the basal epithelial surface and lamina propria cells (Supplementary Figure 4). This localization of NM IIB and NM IIC was not altered in the colonic sections of NM IIA cKO mice (Supplementary Figure 4).
(A) The intestinal permeability of unchallenged NM IIA+/+ and NM IIA cKO mice was examined by measuring the trans-mucosal flux of FITC-dextran. (B) Immunoblotting analysis and selective densitometric quantification of AJ and TJ protein expression in the colonic epithelial scrapes of NM IIA+/+ and NM II cKO animals. (C,D) Immunofluorescence labeling and confocal microscopy of β-catenin (C) and p120 catenin (D) in colonic sections. Arrows show decreased labeling intensity of AJ proteins in the colonic crypts of NM IIA cKO mice. Data is presented as mean ± SE; *P < 0.01. Scale bar, 10 μm.
Colonic sections of control and NM IIA cKO animals were dual-immunolabeled for occludin (red) and claudin-7 (green), and examined by confocal microscopy. Arrows indicate normal labeling of claudin-7 at the intercellular junctions of control mice. Arrowheads point out the cytoplasmic translocation of claudin-7 in the colonic epithelium of NM IIA cKO mice. Scale bar, 10 μm.
Immunofluorescence and fluorescence labeling of NM IIA and F-actin, respectively, in different areas of the colonic epithelium of control and NM IIA cKO mice. Arrows indicate localization of NM IIA in NM IIA+/+ epithelium, whereas arrowheads point to intact F-actin bundles in NM IIA+/+ and NM IIA-depleted colonic mucosa. Scale bar, 10 μm.
Intestinal epithelial specific knockout of NM IIA increased the severity of experimental colitis
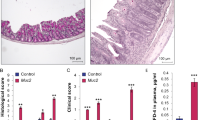
Since disruption of the intestinal epithelial barrier plays an important role during mucosal inflammation we next sought to investigate whether loss of intestinal epithelial NM IIA affects the development of experimental colitis. Administration of a high (5%) dose of dextran sodium sulfate (DSS) induced approximately 80% mortality in NM IIA cKO mice without causing animals’ death in the control group (Fig. 5A). A lower dose of DSS (3%) triggered more severe intestinal disease in NM IIA cKO mice, as manifested by more pronounced body weight loss (Fig. 5B), a significantly higher disease activity index (Fig. 5C), and shortening of the colon (Fig. 5D). DSS administration disrupted intestinal mucosal barriers causing a marked increase in transmucosal passage of FITC-dextran (compare Figs 6A and 2A). Remarkably, DSS-induced mucosal permeability was approximately 5-fold higher in NM IIA cKO animals, as compared to their NM IIA+/+ littermates (Fig. 5A). Histological analysis demonstrated mucosal damage and inflammation in the colonic mucosa of DSS-treated NM IIA+/+ mice that included submucosal edema, crypt hyperplasia, leukocyte infiltration, and focal epithelial erosion (Fig. 6B,C; Supplementary Figure 5). This mucosal injury was much more pronounced in the tissue samples of DSS-exposed NM IIA cKO mice, as characterized by the marked loss of epithelial cells in the distal colon (Fig. 6B,C; Supplementary Figure 5). Consistent with our histology data, TUNEL assay of whole colonic segments (Fig. 6 D,E), and immunoblotting analysis of colonic epithelial scrapes (Supplementary Figure 6), revealed much more pronounced apoptosis in the colonic mucosa of DSS-treated NM II cKO mice, as compared to similarly-treated control animals. Together, this data strongly suggests that loss of intestinal epithelial NM IIA sensitizes mice to DSS-induced colitis.
(A) NM IIA+/+ and NM IIA cKO mice were exposed to 5% DSS in drinking water for 7 days. A Kaplan-Meyer plot shows colitis-induced animal mortality. NM IIA+/+ and NM IIA cKO mice were exposed to 3% DSS in drinking water, or water alone, for 7 days. (B) Body weight, and (C) disease activity index were recorded daily. (D) Colon length was measured at the end of the experiment. Data is presented as mean ± SE. Number of animals in each experimental group is shown in parentheses. *P < 0.01, #P < 0.05
NM IIA+/+ and NM IIA cKO mice were exposed to 3% DSS in drinking water, or water alone, for 7 days. (A) Intestinal permeability was determined by measuring transmucosal flux of FITC-dextran. (B,C) Hematoxylin & eosin staining was used to evaluate epithelial integrity and to calculate the tissue injury index. Changes in the tissue architecture are indicated by letters: e, epithelial erosion; i, leukocyte infiltration; s, submucosal edema. Scale bar, (B) 50 μm. (D,E) Apoptotic cells were visualized using TUNEL assay (red). Nuclear counter-staining (blue) was used to visualize the position of individual cells. Data is presented as mean ± SE. Number of animals in each experimental group is shown in parentheses. *P < 0.01. Scale bar, (D) 10 μm.
Intestinal epithelial specific knockout of NM IIA exacerbated the inflammatory response in colonic mucosa
We next sought to investigate if the increased severity of the disease and more pronounced mucosal damage of DSS-treated NM IIA cKO animals was due to enhanced mucosal inflammation. First, we measured the expression of different proinflammatory cytokines during the early stages of colitis (4 days of DSS administration). Interestingly, quantitative RT-PCR analysis revealed a modest but significant increase in the mRNA levels of proinflammatory cytokines, tumor necrosis factor alpha (TNFα), interleukin (IL-12), and a chemokine, C-X-C motif ligand 5 (CXCL5), in the normal colonic mucosa of NM IIA cKO mice as compared to their NM IIA+/+ littermates (Fig. 7). This further supports our suggestion that a low-scale mucosal inflammation is present in these animals. Moreover, the mRNA expression of virtually all measured cytokines (TNFα, IL-1β, IL-10, IL-12 and IL-17), chemokines (CXCL5, C-C motif ligand 3 (CCL3); keratinocyte-derived chemokine (KC), macrophage inflammatory protein 2 (Mip2) and cyclooxygenase (Cox) 2 was dramatically upregulated in the colonic mucosa of DSS-treated NM IIA cKO mice, as compared to their DSS-exposed controls (Fig. 7).
NM IIA+/+ and NM IIA cKO mice were exposed to either 3% DSS in drinking water, or water alone, for 4 days. Colonic samples were harvested and the expression of different cytokines, chemokines and Cox2 was determined by real-time quantitative RT-PCR. Data is presented as mean ± SE. Number of animals in each experimental group is shown in parentheses. *P < 0.01.
Finally, we sought to determine whether loss of NM IIA affects the recruitment of different classes of leukocytes into inflamed colonic mucosa. We utilized a myeloperoxidase (MPO) activity assay and immunolabeling of F4/80 and CD4 antigens to detect neutrophils, macrophages, and T-lymphocytes respectively. A significant increase in MPO activity was observed in the colonic tissue of unchallenged NM IIA cKO mice (Fig. 8A). Additionally, NM IIA cKO animals showed much higher induction of MPO activity after 7 days of DSS administration. Similarly, DSS colitis in NM IIA cKO mice was accompanied by a more pronounced mucosal recruitment of macrophages and CD4-positive T lymphocytes (Fig. 8B,C). Together this data provides further evidence of low-scale mucosal inflammation in unchallenged NM IIA cKO mice, and reveals the enhanced inflammatory response of these animals during DSS-induced colitis.
Colonic samples of NM IIA+/+ and NM IIA cKO mice were collected on day 7 of 3% DSS or water administration. (A) MPO activity was measured as a marker for neutrophil infiltration. (B,C) Macrophages and T cells were visualized by immunolabeling their specific cellular markers, F4/80, and CD4, respectively (red). Nuclear counter-staining (blue) was used to visualize the position of individual cells. Data is presented as mean ± SE. Number of animals in each experimental group is shown in parentheses. *P < 0,01, #P < 0,05. Scale bar, 10 μm.
Intestinal epithelial specific knockout of NM IIA enhanced the expression of transforming growth factor-beta and the accumulation of mucosal IgA
The persistence of low-scale mucosal inflammation, without the development of spontaneous colitis observed in NM IIA cKO mice, suggests the existence of a protective compensatory response in their intestinal mucosa. A protective immune response that involves transforming growth factor beta (TGF-β) driven production of IgA was recently described in mice with total JAM-A knockout42. Therefore, we sought to investigate if a similar compensatory response exists in NM IIA cKO mice. Quantitative RT-PCR analysis of colonic tissue demonstrated a more than 3 fold increase in TGF-β expression in unchallenged NM IIA cKO mice, as compared to NM IIA+/+ littermates (Fig. 9A). Moreover, immunofluorescence analysis revealed an accumulation of IgA in the NM IIA-depleted intestinal mucosa (Fig. 9B,C). This data suggests that upregulation of TGF-β and IgA expression may protect the animals from spontaneous colitis under ‘leaky’ intestinal epithelial barrier conditions.
(A) TGF-β mRNA expression in the colonic tissue of unchallenged NM IIA+/+ and NM IIA cKO mice was determined using real-time RT-PCR analysis. (B) IgA (green) was visualized in colonic sections by immunolabeling and confocal microscopy. Nuclear counter-staining (blue) was used to visualize the position of individual cells. Data is presented as mean ± SE (n = 5); *P < 0,01. Scale bar, 10 μm.
Discussion
NM II motors are essential regulators of cellular homeostasis and tissue integrity, playing multiple roles in cell polarity, division, motility, and mechanotransduction14,15. The predominant body of studies focuses on the activity and regulation of mammalian NM II in cultured cells, in vitro12,13,14,15,32. However, physiological functions of these motor proteins in different tissues remain poorly understood. The present study provides the first evidence that NM IIA acts as a positive regulator of the intestinal epithelial barrier in normal gut and suppresses intestinal mucosal inflammation in vivo. We successfully generated a selective knockout of NM IIA in the intestinal epithelium (Fig. 1; Supplementary Figure 1). Characterization of unchallenged NM IIA cKO animals revealed several important phenotypes that included increased mucosal permeability (Fig. 2A), decreased expression and mislocalization of some junctional proteins (Figs 2B–D and 3), loss of mucin granules in Goblet cells (Fig. 1D), and the development of low-scale mucosal inflammation (Figs 1B,C, 7 and 8A). The intestinal epithelial phenotypes of NM IIA cKO mice appear to be relatively mild and did not involve global disruption of AJ/TJ integrity or abnormal organization of the perijunctional cytoskeleton. This is consistent with our previous results obtained in NM IIA-depleted cultured intestinal epithelial cell monolayers18. A partial preservation of AJ and TJ structure in NM IIA-depleted intestinal epithelium most likely reflects the compensatory effects of the remaining NM II isoforms, most notably, NM IIC. Indeed, NM IIC was found to account for up to 20% of total NM II in the intestinal epithelium33 and it is enriched at intestinal epithelial apical junctions in vitro18 and in vivo (Supplementary Figure 4).
The observed decreased expression of β-catenin and p120 catenin in the colonic mucosa of NM IIA cKO mice is unusual for two reasons. First, it is not paralleled by the decreased expression or altered localization of E-cadherin (Fig. 2B; Supplementary Figure 3), thereby contrasting with the reported loss of E-cadherin in p120-depleted intestinal epithelial cells43 and tissue44. However NM IIA cKO mice do not show a complete loss of intestinal epithelial p120 catenin, and the remaining pool of this protein could be sufficient to stabilize E-cadherin. Second, the decreased expression of β-catenin and p120-catenin appears to be a specific effect of NM IIA knockout in vivo, since the expression of these AJ scaffolds was not changed in intestinal epithelial cells after either siRNA-mediated knockdown or CRISPR/Cas9-mediated knockout of NM IIA in vitro18 (and data not shown). One can suggest, therefore, that downregulation of β-catenin and p120 catenin could be indirect effects of NM IIA depletion that can be linked to the disruption of the gut barrier and increased exposure to luminal microbiota.
Despite having a defective intestinal epithelial barrier, NM IIA cKO mice did not develop spontaneous colitis. We believe this could be explained by the development of a protective mechanism, similar to the mechanism recently described in JAM-A knockout mice41,42. NM IIA cKO and JAM-A knockout mice showed similar physiological and biochemical abnormalities that include: (i) leaky gut barrier; (ii) lymphoid aggregates in the colonic mucosa; (iii) increased expression of colonic TGF-β; (iv) and the accumulation of intestinal mucosal IgA. A comprehensive genetic and immunological analysis of JAM-A mice concluded that they have an activated CD4 T-cell response resulting in TGF-β-dependent secretion of IgA. This limits bacterial translocation and intestinal mucosal inflammation under conditions of a compromised intestinal epithelial barrier42. Our data suggests that a similar compensatory mechanism may limit the inflammatory response in the intestinal mucosa of unchallenged NM IIA cKO animals (Fig. 9).
The present study reveals a key role of NM IIA in attenuating mucosal inflammation and tissue injury in DSS-challenged mice (Figs 5, 6, 7, 8; Supplementary Figures 5 and 6). This anti-inflammatory role is likely to involve the barrier-stabilizing activity of this cytoskeletal motor, since increased mucosal inflammation correlated with a more dramatic breakdown of gut barrier in NM IIA cKO animals (Fig. 6A). It is unlikely that the more severe mucosal damage and inflammation in NM IIA cKO mice reflect the increased sensitivity of epithelial cells to DSS toxicity. Indeed NM II inhibition was shown to have prosurvival effects on mammalian stem cells and intestinal enteroids45,46. Furthermore, we did not observe increased death of NM IIA knockout HT-29 cells after direct exposure to 3% DSS in vitro (data not shown).
Our data purporting the anti-inflammatory role of intestinal epithelial NM IIA is in variance with a recent report suggesting that inhibition of intestinal NM IIA alleviates DSS-induced colitis46. These authors attenuated NM IIA activity by using either a pharmacological NM II inhibitor, blebbistatin, or via monoallelic deletion of NM IIA in the intestinal epithelium (NM IIA flox/+/Villin-cre)46. However, the effects of blebbistatin on DSS colitis are difficult to interpret, since this inhibitor directly blocks leukocyte infiltration and activity in the intestinal mucosa. Furthermore, monoallelic NM IIA deletion in mice neither decreased expression of this protein, nor did it alter animal responsiveness to DSS in our experiments (data not shown). We believe that NM IIA cKO animals developed in our study represent the best tool to investigate the in vivo functions of this cytoskeletal motor in the intestinal epithelium.
The increased permeability of normal and inflamed intestinal mucosa observed in NM IIA cKO mice challenges the current dogma that only NM IIA activation drives opening of the gut barrier under physiological conditions and during intestinal inflammation. Taken together, our data and previous studies indicate that a balanced, intermediate activity of NM IIA is essential for the integrity of the normal gut barrier. Both excessive activation and inactivation of NM II motor should increase intestinal permeability. While the external stimuli and signaling events that activate intestinal epithelial NM II have been intensively investigated1,9,47,48, little is known about how this motor could be inhibited in gastrointestinal diseases. A number of different mechanisms controlling the assembly and activity of NM II motors have been described in vitro. These mechanisms include regulation of expression, chaperon-assisted folding, heavy chain phosphorylation, and associations with multiple myosin-binding proteins15,32. Further study is required to elucidate how these mechanisms control NM IIA functions in the gut under physiological conditions and during gastrointestinal diseases.
Materials and Methods
Antibodies and other reagents
The following primary monoclonal (mAb) and polyclonal (pAb) antibodies were used to detect cytoskeletal, junctional, and leukocyte proteins: anti-p120-catenin and E-cadherin, Ly-6 and CD4 mAbs (BD Biosciences, San Jose, CA); anti-occludin, JAM-A, ZO-1, Claudin-1 and 7 pAbs, and Claudin-4 mAb (Life Technologies, Waltham, MA); anti-total actin (clone C4) mAb (EMD Millipore, Billerica, MA); anti-GAPDH (14C10) mAb and active caspase 3 pAb (Cell Signaling, Beverly, MA); anti-β-catenin pAb (Sigma-Aldrich, Saint Lois, MO); anti-α-catenin mAb (Abcam, Cambridge, MA); anti-NM IIA, NM IIB and NM IIC pAbs (Covance, Princeton, NJ); F4/80 mAb (Bio-Rad Laboratories, Hercules, CA); goat anti-E-cadherin pAb (R&D Systems, Minneapolis, MN); anti- lysozyme pAb (Santa Cruz, Dallas, TX); FITC conjugated rat anti-mouse IgA (eBioscience, San Diego, CA). Alexa Fluor-488-conjugated donkey anti-rabbit and donkey anti-goat, Alexa Fluor-555-conjugated donkey anti-mouse and goat anti-rat secondary antibodies, and Alexa Fluor-488 and 555-labeled phalloidin were obtained from Life Technologies. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were acquired from Bio-Rad Laboratories. All other chemicals were obtained from Sigma-Aldrich.
Animals
In order to establish a conditional knockout of NM IIA in the intestinal epithelium, NM II Aflox/flox mice on a C57BL/6/129/Sv background49 were crossed with villin-Cre animals (Jackson Laboratory, stock #004586). In these villin-Cre mice, a 12.4 kb fragment of mouse villin 1 promoter directs transgenic recombination in both the small intestine and the colon50. The animal colony was maintained under pathogen-free conditions in the vivarium of VCU Medical Center. Standard feed and tap water were available, ad libitum. The mouse room was on a 12 h light/dark cycle (lights on at 7:00 A.M.). At the beginning of experiments, mice weighed 18–25 g, and there was no meaningful difference between the body masses of mice of different genotypes. All procedures were conducted under an animal research protocol (AD10000458) approved by the Virginia Commonwealth University Animal Care and Use Committee in accordance with the National Institutes of Health Animal Care and Use Guidelines.
Induction and characterization of dextran sulfate sodium (DSS) colitis
Experimental colitis was induced in 8–10 week old NM II Aflox/flox/villin Cre+ mice (abbreviated as NM IIA cKO) by administering either a 5% or 3% (w/v) solution of DSS (molecular weight 40 kDa; MP Biomedicals, Santa Ana, CA) in drinking water, ad libitum. Either NM IIAflox/flox or villin-Cre only littermates were used as controls (referred as NM IIA+/+). Unchallenged animals received tap water. Both male and female mice were used at roughly equal numbers in this study. The animals were weighed daily and monitored for signs of intestinal inflammation. The disease activity index was calculated as previously described, by averaging numerical scores of body weight loss, stool consistency, and intestinal bleeding51. With regards to body weight, no weight loss was scored as 0, loss of 1–5% was scored as 1, 5–10% weight loss as 2, 10–15% as 3, and more than 15% weight loss was scored as 4. For stool consistency, well-formed pellet was scored as 0, soft and semi-formed stool as 2, and liquid stool or diarrhea scored as 4. For intestinal bleeding, no blood was scored as 0, hemoccult-positive stool as 2, and gross rectal bleeding was scored as 4. On day 7 of DSS administration, animals were euthanized and their colonic tissue was separated into several segments. The samples were either fixed in 4% paraformaldehyde (PFA), snap frozen in liquid nitrogen, or embedded in OCT and frozen for subsequent histological and biochemical examination. PFA-fixed samples were paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E). The tissue injury index was calculated based on microscopic examination of H&E sections, as previously described52. The index represents the sum of individual scores reflecting leukocyte infiltration, crypt hyperplasia/inflammation, and epithelial erosion.
Measurement of epithelial barrier permeability in vivo
In vivo permeability assay was performed in NM II A cKO and NM IIA+/+ animals receiving either 3% DSS for 7 days or water. Animals were gavaged with 4,000 Da FITC-labeled dextran (60 mg/100 g body weight) and euthanized 3 h later for blood collection via cardiac puncture. Blood serum was obtained by centrifugation, and FITC fluorescence intensity was measured using a Victor3 V plate reader (Perkin Elmer, Waltham, MA) with excitation and emission wavelengths at 485 and 544 nm, respectively. The value of FITC-dextran-free serum was subtracted from each measurement. The concentration of FITC-dextran in blood serum was calculated using SigmaPlot v12.5 software, based on a plotted standard curve prepared via serial dilutions of the stock solution of FITC-dextran in phosphate buffered saline (PBS).
Immunoblotting analysis
Mice were euthanized, colonic and ileal segments were harvested, longitudinally opened, and washed with ice-cold PBS. Intestinal epithelium was collected by scraping the exposed interior with glass slides, then snap frozen in liquid nitrogen for further analysis. Intestinal epithelial scrapes were lysed and homogenized in RIPA buffer containing a protease inhibitor cocktail and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich). Samples were diluted with 2x SDS sample loading buffer and boiled. SDS-polyacrylamide gel electrophoresis was conducted using standard protocols with an equal amount of total protein loaded per lane (10 or 20 μg), followed by immunoblotting on nitrocellulose membrane. Protein expression was quantified via densitometry using Image J software (National Institutes of Health, Bethesda MD).
Quantitative real-time RT-PCR
Total RNA was isolated from the whole colonic segments of NM IIA+/+ and NM IIA cKO animals using an RNeasy mini kit (QIAGEN, Valencia, CA) followed by DNase treatment to remove genomic DNA. Total RNA (1 μg) was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative real-time RT-PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories) and a 7900HT Fast Real-time PCR System (Applied Biosystems, Foster City, CA). The primer sequences are listed in the Supplemental Table 1. The threshold cycle number (Ct) for specific genes of interest and a housekeeping gene were determined based on the amplification curve representing a plot of the fluorescent signal intensity versus the cycle number. Relative expression of each gene was calculated by a comparative Ct method that is based on the inverse proportionality between Ct and the initial template concentration (2−ΔΔCt), as previously described53. This method is based on two-step calculations of ΔCt = Cttarget gene − CtGAPDH and ΔΔCt = ΔCte − ΔCtc, where index e refers to the sample from any DSS or water-treated NM II A cKO, or NM IIA+/+ mice, and index c refers to the sample from a water-treated NM IIA+/+ animal assigned as an internal control.
Immunofluorescence labeling, TUNEL assay, confocal microscopy and histochemistry
Colonic frozen sections were fixed with 95% ethanol to visualize junctional proteins, NM II isoforms, and leukocyte markers. PFA fixed and paraffin embedded sections were used for F-actin and IgA labeling. Following standard deparaffinization and antigen retrieval, sections were blocked for 60 minutes in Hanks HEPES-buffered salt solution containing 1% bovine serum albumin, followed by a 60 min incubation with primary antibodies. Samples were then washed and incubated with Alexa dye-conjugated secondary antibodies for 60 minutes, rinsed with blocking buffer, and mounted on slides with ProLong Antifade mounting reagent with or without DAPI (Life Technologies). F-actin was visualized by 60 min labeling with Alexa-555-labeled phalloidin. TUNEL assay was performed using an ApopTag Fluorescein In Situ Apoptosis Detection Kit (EMD Millipore), according to the manufacturer’s instructions. Labeled cell monolayers and tissue sections were imaged using a Zeiss LSM 700 Laser Scanning Confocal Microscope (Carl Zeiss Microscopy LCC, Peabody, MA). The Alexa Fluor 488 and 555 signals were acquired sequentially, in frame-interlace mode, to eliminate cross talk between channels. Images were processed using Zen 2011 software and Adobe Photoshop. To quantify the results of tissue TUNEL assay, T-cell marker (CD4) and macrophage marker (F4/80) labeling, signal intensity was measured on the mucosal surface and crypt areas of each animal’s sample. Mean values were calculated by averaging signal intensities obtained from the tissue samples of 5–7 different animals from each experimental group. The animal numbers are presented in the figures. Histochemical visualization of Goblet cells was performed using a combined Periodic Acid-Shiff and Alcian Blue staining kit (Sigma-Aldrich), according to the manufacturer’s instructions. Stained tissue sections were examined using a light microscope (Olympus BX41, Japan). Mucin granules were counted manually in blind fashion and, data are presented as number of granules per microscopic field.
Myeloperoxidase (MPO) activity assay
MPO activity in the colons of unchallenged and DSS colitis induced animals was determined fluorometrically using a kit from Sigma-Aldrich, as described by the manufacturer. Briefly, frozen colonic samples were homogenized in MPO assay buffer. After brief centrifugation, supernatants were assayed for MPO activity using a fluorigenic substrate, aminophenyl fluorescein, and analysis on the Victor3 V plate reader with excitation and emission wavelengths 485 and 544 nm, respectively. A standard curve was created using serial dilutions of known concentrations of the kit enzyme. One unit of MPO activity was determined as the amount of the enzyme that oxidizes the MPO substrate to yield 1.0 μmole of fluorescein per minute at room temperature.
Statistical analysis
Data are given as mean ± SEM. The statistical significance of the difference between 2 sets of data was evaluated by the two tailed unpaired Student’s T-test. Differences among three or more groups were tested for statistical significance using one way ANOVA (SigmaPlot 12.5 package). Statistical significance was accepted at P < 0.05.
Additional Information
How to cite this article: Naydenov, N. G. et al. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci. Rep. 6, 24161; doi: 10.1038/srep24161 (2016).
References
Marchiando, A. M., Graham, W. V. & Turner, J. R. Epithelial barriers in homeostasis and disease. Ann Rev Pathol 5, 119–144 (2010).
Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mole Life Sci 70, 631–659 (2013).
Ivanov, A. I. & Naydenov, N. G. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol 303, 27–99 (2013).
Takeichi, M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nature reviews Mol Cell Biol 15, 397–410 (2014).
Troyanovsky, S. Adherens junction assembly. Subcell Biochem 60, 89–108 (2012).
Anderson, J. M. & Van Itallie, C. M. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1, a002584 (2009).
Koval, M. Differential pathways of claudin oligomerization and integration into tight junctions. Tissue Barriers 1, e24518 (2013).
Lu, Z., Ding, L., Lu, Q. & Chen, Y.-H. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers 1, e24978 (2013).
McCole, D. F. Phosphatase regulation of intercellular junctions. Tissue Barriers 1, e26713 (2013).
Quiros, M. & Nusrat, A. RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Sem Cell Dev Biol 36, 194–203 (2014).
Ivanov, A. I. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci 13, 6662–6681 (2008).
Rodgers, L. S. & Fanning, A. S. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton 68, 653–660 (2011).
Ivanov, A. I., Parkos, C. A. & Nusrat, A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J. Pathol 177, 512–524 (2010).
Heissler, S. M. & Manstein, D. J. Nonmuscle myosin-2: mix and match. Cell Mol Life Sci 70, 1–21 (2013).
Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nature reviews Mol Cell Biol 10, 778–790 (2009).
Al-Sadi, R., Ye, D., Dokladny, K. & Ma, T. Y. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 180, 5653–5661 (2008).
Clayburgh, D. R. et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo . J Clin Invest 115, 2702–2715 (2005).
Ivanov, A. I. et al. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. Plos one 2, e658 (2007).
Ivanov, A. I., Hunt, D., Utech, M., Nusrat, A. & Parkos, C. A. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 16, 2636–2650 (2005).
Ivanov, A. I., McCall, I. C., Parkos, C. A. & Nusrat, A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell 15, 2639–2651 (2004).
Shen, L. et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119, 2095–2106 (2006).
Utech, M. et al. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16, 5040–5052 (2005).
Zolotarevsky, Y. et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123, 163–172 (2002).
Shen, L., Weber, C. R., Raleigh, D. R., Yu, D. & Turner, J. R. Tight junction pore and leak pathways: a dynamic duo. Ann Rev Physiol 73, 283–309 (2011).
Su, L. et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 145, 407–415 (2013).
Su, L. et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, 551–563 (2009).
Feighery, L. M. et al. Myosin light chain kinase inhibition: correction of increased intestinal epithelial permeability in vitro . Pharm Res 25, 1377–1386 (2008).
Heissler, S. M. & Sellers, J. R. Four things to know about myosin light chains as reporters for non-muscle myosin-2 dynamics in live cells. Cytoskeleton 72, 65–70, (2015).
Chan, W. et al. Myosin II regulatory light chain is required for trafficking of bile salt export protein to the apical membrane in Madin-Darby canine kidney cells. J Biol Chem 280, 23741–23747, (2005).
Szymanski, P. T. & Goyal, R. K. Calponin binds to the 20-kilodalton regulatory light chain of myosin. Biochemistry 38, 3778–3784, (1999).
Xu, J. et al. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins. Nature immunol 9, 880–886, (2008).
Beach, J. R. & Hammer, J. A., 3rd . Myosin II isoform co-assembly and differential regulation in mammalian systems. Experimental cell research 334, 2–9 (2015).
Babbin, B. A. et al. Non-muscle myosin IIA differentially regulates intestinal epithelial cell restitution and matrix invasion. Am J Pathol 174, 436–448 (2009).
Ivanov, A. I., Samarin, S. N., Bachar, M., Parkos, C. A. & Nusrat, A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol 10, 36–43, (2009).
Wald, F. A. et al. Aberrant expression of the polarity complex atypical PKC and non-muscle myosin IIA in active and inactive inflammatory bowel disease. Virchows Archiv 459, 331–338 (2011).
Conti, M. A., Even-Ram, S., Liu, C., Yamada, K. M. & Adelstein, R. S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem 279, 41263–41266 (2004).
de Valliere, C. et al. G Protein-coupled pH-sensing Receptor OGR1 Is a Regulator of Intestinal Inflammation. Inflamm Bowel Dis 21, 1269–1281, (2015).
Matharu, K. S. et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 137, 1380–1390 (2009).
Neurath, M. F., Fuss, I., Kelsall, B. L., Stuber, E. & Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 182, 1281–1290 (1995).
Schumann, M. et al. CCR7 deficiency causes diarrhea associated with altered ion transport in colonocytes in the absence of overt colitis. Mucosal Immunol 5, 377–387 (2012).
Laukoetter, M. G. et al. JAM-A regulates permeability and inflammation in the intestine in vivo . J Exp Med 204, 3067–3076 (2007).
Khounlotham, M. et al. Compromised intestinal epithelial barrier induces adaptive immune compensation that protects from colitis. Immunity 37, 563–573 (2012).
Naydenov, N. G. et al. A membrane fusion protein alphaSNAP is a novel regulator of epithelial apical junctions. PloS one 7, e34320 (2012).
Smalley-Freed, W. G. et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest 120, 1824–1835 (2010).
Walker, A. et al. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nature Commun 1, 71 (2010).
Zhao, B. et al. The non-muscle-myosin-II heavy chain Myh9 mediates colitis-induced epithelium injury by restricting Lgr5+ stem cells. Nature Commun 6, 7166 (2015).
Barreau, F. & Hugot, J. P. Intestinal barrier dysfunction triggered by invasive bacteria. Curr Opin Microbiol 17, 91–98 (2014).
Cunningham, K. E. & Turner, J. R. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci 1258, 34–42 (2012).
Jacobelli, J. et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nature immunol 11, 953–961 (2010).
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277, 33275–33283 (2002).
Rhee, L. et al. Expression of TNFAIP3 in intestinal epithelial cells protects from DSS- but not TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 303, G220–227 (2012).
Kennedy, R. J. et al. Interleukin 10-deficient colitis: new similarities to human inflammatory bowel disease. Br J Surg 87, 1346–1351 (2000).
Ivanov, A. I., Pero, R. S., Scheck, A. C. & Romanovsky, A. A. Prostaglandin E(2)-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul, Integr Comp Physiol 283, R1104–1117 (2002).
Acknowledgements
Services in support of this study were provided by the VCU Massey Cancer Center, supported in part with funding from NIH-NCI core grant P30CA016059. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from the NIH-NINDS Center core grant 5P30NS047463. This work was supported by National Institute of Health grants RO1 DK083968, R01 DK084953 to A.I.I. and by the Crohn’s and Colitis Foundation of America research fellowship award 254881 to N.G.N.
Author information
Authors and Affiliations
Contributions
A.I.I. conceived the study, supervised the project and wrote the manuscript. N.G.N. performed experiment, analyzed and interpreted data and prepared figures. A.F., G.H. and D.W. performed experiment and acquired data. R.S.A. and M.A.C. provided NM IIA floxed animals and helped with manuscript preparation. J.F.K. was involved in the design, data analysis and interpretation of the experimental colitis study. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Naydenov, N., Feygin, A., Wang, D. et al. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci Rep 6, 24161 (2016). https://doi.org/10.1038/srep24161
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24161
This article is cited by
-
Claudin-23 reshapes epithelial tight junction architecture to regulate barrier function
Nature Communications (2023)
-
Intestinal Barrier Dysfunction in Inflammatory Bowel Disease: Underpinning Pathogenesis and Therapeutics
Digestive Diseases and Sciences (2023)
-
Intestinal Claudin-7 deficiency impacts the intestinal microbiota in mice with colitis
BMC Gastroenterology (2022)
-
GPR120 prevents colorectal adenocarcinoma progression by sustaining the mucosal barrier integrity
Scientific Reports (2022)
-
Myosin IIA Regulated Tight Junction in Oxygen Glucose-Deprived Brain Endothelial Cells Via Activation of TLR4/PI3K/Akt/JNK1/2/14-3-3ε/NF-κB/MMP9 Signal Transduction Pathway
Cellular and Molecular Neurobiology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.