Abstract
The non-toxic and wide bandgap material TiO2 is explored as an n-type buffer layer on p-type Cu(In,Ga)Se2 (CIGS) absorber layer for thin film solar cells. The amorphous TiO2 thin film deposited by atomic layer deposition process at low temperatures shows conformal coverage on the CIGS absorber layer. Solar cells from non-vacuum deposited CIGS absorbers with TiO2 buffer layer result in a high short-circuit current density of 38.9 mA/cm2 as compared to 36.9 mA/cm2 measured in the reference cell with CdS buffer layer, without compromising open-circuit voltage. The significant photocurrent gain, mainly in the UV part of the spectrum, can be attributed to the low parasitic absorption loss in the ultrathin TiO2 layer (~10 nm) with a larger bandgap of 3.4 eV compared to 2.4 eV of the traditionally used CdS. Overall the solar cell conversion efficiency was improved from 9.5% to 9.9% by substituting the CdS by TiO2 on an active cell area of 10.5 mm2. Optimized TiO2/CIGS solar cells show excellent long-term stability. The results imply that TiO2 is a promising buffer layer material for CIGS solar cells, avoiding the toxic CdS buffer layer with added performance advantage.
Similar content being viewed by others
Introduction
Among all thin film technologies, solar cells based on Cu(In,Ga)Se2 (CIGS) absorbers yield the best performance1. Recently, ZSW has reported its record CdS/CIGS solar cell with efficiency as high as 21.7%2. So far, CIGS cells with CdS buffer layer deposited by chemical bath deposition (CBD) have resulted in the highest conversion efficiencies. This is due to a suitable band alignment and in-situ surface passivation during CBD. The disadvantages of CdS, however, are parasitic absorption in the blue part of the solar spectrum and the toxicity of Cd3. Alternative buffer layers for CIGS which have been successfully employed are comprised of binary or multinary Zn(S,O,OH) and ZnMgO4,5,6,7. The aforementioned compounds have tunable band gaps larger than 3.5 eV and can form hole blocking contacts to CIGS8,9. Recently, ZSW presented a 21% efficient CIGS cell with zinc oxysulfide and zinc magnesium oxide layers substituting the conventional CdS buffer layer and ZnO front contact, respectively10,11. However, compound buffer layers consisting of Zn(S,O,OH) can have some disadvantages such as complicated reaction mechanism12, high resistivity (ZnS13) and light soaking effects14,15, presenting a potential cell reliability problem.
CIGS thin films deposited by vacuum processes such as co-evaporation and sputtering yield the highest efficiencies to date but require complex multistep processes16,17. Lower cost processing techniques would allow an important contribution to reduce the cost of photovoltaics. Non-vacuum deposition processes based on chemical precursor solution or nanoparticle printing offer the possibility to reduce the manufacturing costs and give the flexibility for high throughput large area upscaling. An efficiency as high as 17.1% was demonstrated by printing a nanoparticle ink followed by rapid thermal processing to form CIGS18.
We chose non-vacuum deposited CIGS to demonstrate that TiO2 can be utilized as a selective electron contact on CIGS solar cells leading to a clear enhancement in photocurrent without compromising open-circuit voltage (Voc). Our results demonstrate that TiO2 is a promising candidate to successfully substitute the toxic CdS buffer layer. In this study, TiO2 thin films were deposited on printed nanoparticle based CIGS absorber layers by using the atomic layer deposition (ALD) technique. The ALD process provides good and uniform coverage as well as excellent thickness control19 on the surface of the CIGS absorber layer. To our knowledge, this is the first time that the wide band gap material TiO2 has been used successfully as alternative buffer layer in single junction planar CIGS solar cells.
Results and Discussion
A sketch of the fabrication flow with the corresponding deposition methods is shown in Fig. 1(a). CIGS layers were deposited by non-vacuum printing of metal oxide powders on Cr/Mo coated stainless steel foil substrates (thickness 75 μm) followed by annealing in reducing H2 atmosphere and finally selenization in H2Se at 500 °C. As n-type buffer layer, either CdS or TiO2 is deposited by chemical bath deposition or ALD, respectively, followed by sputtering of the transparent electron contact indium tin oxide (ITO). The photograph in Fig. 1(b), shows a complete device with Ag grid fingers to facilitate electron extraction, manually scribed to cell sizes of 3.5 × 3.5 mm2, resulting in an active area of 10.5 mm2.
(a) Process schematic of the TiO2/CIGS solar cell, (b) photograph of the TiO2/CIGS solar cell device, (c) SEM cross-sectional view of a fully fabricated TiO2/CIGS solar cell prepared by FIB, (d) high resolution SEM cross-sectional view of ITO/TiO2/CIGS. The sample was coated with Pt to protect it during the FIB cut.
Figure 1(c) presents a focused ion beam prepared cross-sectional scanning electron microscope (SEM) image of the final TiO2/CIGS solar cell. Due to the reaction of Mo with H2Se, approximately 850 nm MoSe2 is formed at the interface of CIGS with Mo. The MoSe2 layer not only provides quasi-ohmic contact between Mo and CIGS but also builds up a backside field, which benefits hole transportation20. As typically observed in a two-step non-vacuum coating and selenization process, a bi-layer comprising a dense CIGS layer on the top and a porous nanocrystalline bottom layer forms. The dense upper layer is indium-rich (CuInSe2 (CIS)) while the bottom layer with small grains is gallium-rich (CuGaSe2) as measured by X-ray photoelectron spectroscopy (XPS) depth profiling (see supporting information Figure S1). The high magnification SEM image in Fig. 1(d) shows the conformal coverage of TiO2 on the CIGS absorber layer. The thickness of the amorphous TiO2 film was measured to be 15 nm.
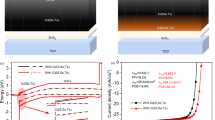
In our earlier work we characterized the structural and electronic properties of the TiO2 thin films deposited by ALD21. Atomic force microscopy and Raman spectroscopy revealed that TiO2 films grown at 120 °C are smooth and in an amorphous phase. XPS revealed close to stoichiometric TiO2 with a work function of 4.5 eV and the valence band maximum located at 7.4 eV. The band gap is 3.4 eV. In Fig. 2 we show a comparison of the schematic energy band diagrams of the ITO/TiO2/CIGS solar cell and the ITO/CdS/CIGS reference cell simulated by SCAPS under equilibrium conditions22. Please note, due to Ga segregation towards the back (compare supporting information, Figure S1), we simulate the buffer/absorber interface with CIS instead of CIGS. The relevant parameters for the energy band diagram simulation are listed in the table included in Fig. 2. The band diagram shows the p-CIS in contact with n-TiO2/ITO and n-CdS/ITO, respectively, to form the pn-junction. Both the TiO2 and CdS form a large barrier for majority carriers (holes) due to the large valence band offset with the CIS absorber. The positive conduction band offset (spike) for the TiO2/CIS interface is larger than for the CdS/CIS reference cell due to a lower electron affinity of TiO2 (4.0 eV21) compared to CdS (4.3 eV23). The SCAPS simulation of the TiO2/CIGS sample hypothesizes an inverted surface which might help to reduce recombination at the TiO2/CIGS interface. Further experiments are needed to verify this simulation.
Schematic band diagrams of the CIS/TiO2/ITO (black line) and CIS/CdS/ITO (blue line) solar cells simulated with SCAPS.
The table provides the basic input parameters for the simulation where EC, EV, EF, Eg and EA are the conduction band minimum, valence band maximum, Fermi energy, band gap and electron affinity, respectively.
To optimize the device performance, the influence of the TiO2 deposition temperature and thickness on Voc and short-circuit current density (Jsc) were investigated (Fig. 3(a,b), respectively). The parameters obtained under optimized conditions for the CdS reference cell are given as dashed lines. When increasing the TiO2 deposition temperature, the thickness was fixed to 15 nm. As shown in Fig. 3(a), the Voc of the TiO2/CIGS solar cells rises from 412 mV to 431 mV as the deposition temperature increases from 110 °C to 130 °C, which is above the Voc of the CdS/CIGS reference cell (416 mV). A possible reason for the Voc enhancement might be elemental interdiffusion at the TiO2/CIGS interface which could lead to the formation of an inverted surface accompanied by a drop in interface recombination. The Jsc of the TiO2/CIGS solar cells reaches its maximum of 38.9 mA/cm2 at a TiO2 deposition temperature of 120 °C. A further temperature increase leads to both Voc and Jsc drop to as low as 385 mV and 34.7 mA/cm2 at 180 °C, respectively. As it was found earlier by Yin et al.21 the TiO2 film deposited at 120 °C is in an amorphous phase and shows a smooth morphology. As the temperature increases the phase changes from amorphous to nanocrystalline accompanied by a rougher morphology21, which possibly explains the drop in all photovoltaic parameters. However, at elevated deposition temperature not only the phase of the TiO2 might play an important role for the pn-junction formation but also possible ion diffusion at the TiO2/CIGS interface or within the CIGS which should be the subject of further studies.
To study the influence of the TiO2 thickness, the deposition temperature was fixed at 120 °C (Fig. 3(b)). The Voc and Jsc of TiO2/CIGS solar cells increase with TiO2 thickness up to 10 nm. A maximum Voc of 436 mV and Jsc of 39.1 mA/cm2 are measured for 7 and 10 nm TiO2, respectively. As the TiO2 thickness exceeds 15 nm the Jsc drops significantly due to residual light absorption while the Voc remains at the CdS/CIGS reference level.
Inspection of the external quantum efficiency (EQE) curves of the TiO2/CIGS and CdS/CIGS devices in Fig. 4(a) reveal a significant photocurrent gain in the wavelength range between 300 and 630 nm. Between 630 and 1060 nm the EQE of the CdS/CIGS reference device is slightly higher, this however can be correlated to a reduced light in-coupling caused by higher reflectance losses (R, presented as 1-R) in the TiO2/CIGS device seen in Fig. 4(a). Higher reflectance losses in the TiO2/CIGS solar cell might be caused by a slightly non-ideal total oxide thickness that allows for constructive interference of the reflected light but can be circumvented by applying an anti-reflection coating. The minimum band gaps of the CIGS absorbers are 0.95 eV (with TiO2) and 0.96 eV (with CdS) as extracted from the sharp EQE cut-off at high wavelengths. In Fig. 4(b) we show the absorption (A%) of the individual window layers TiO2 (10 nm), CdS (50 nm) and ITO (50 nm) as well as their combinations TiO2/ITO and CdS/ITO deposited on quartz glass substrates. Both, the absorption onsets of TiO2 and CdS match well with their band gaps of 3.4 eV and 2.4 eV, respectively. The CdS/ITO bilayer shows high absorption for wavelengths <600 nm as compared to the TiO2/ITO bilayer accounting for the significant gain in photocurrent for the latter device stack as previously discussed.
The current-voltage (J-V) characteristics of the optimized TiO2/CIGS device and a reference CdS/CIGS device are shown in Fig. 4(c) (corresponding cell parameters are summarized in Table 1). The best performance, mainly due to a significant photocurrent gain of 2.0 mA/cm2 was obtained with 10 nm amorphous TiO2 deposited at 120 °C. The gain in photocurrent seen in the TiO2/CIGS device can be even higher by optimizing the antireflection coating as indicated by the 1-R measurements (Fig. 4(a)). The fill factor (FF) of the reference CdS/CIGS solar cell is slightly higher than the FF of the TiO2/CIGS cell, which are 61.4% and 59.6%, respectively. This difference is caused by the higher series resistance of 2.8 Ohm cm2 (TiO2/CIGS) as compared to 1.9 Ohm cm2 (CdS/CIGS) probably related to a larger resistivity of the TiO2 thin film. This could be mitigated in the future by doping the TiO2 layer. It is assumed that the porous CIGS absorber (compare Fig. 1(c)) gives rise to the relatively high series resistance observed in all devices. The Voc of the TiO2/CIGS solar cell (426 mV) does not suffer from a higher conduction band offset presented in the simulated band diagram (Fig. 2) and is marginaly higher than the 416 mV measured in the CdS/CIGS solar cell. Finally, the TiO2/CIGS device shows an active area efficiency of 9.9%, which is slightly better than the efficiency of the CdS/CIGS reference device (9.5%).
To asses the long-term stability of the TiO2/CIGS (15 nm TiO2 deposited at 120 °C) and CdS/CIGS solar cells, J-V curves were remeasured after 9 months and are presented in Fig. S2 (supporting information) and are summarized in Table 1. Both devices show degradation over time. The TiO2/CIGS solar cell only suffers FF degradation which fully recovers under light soaking within 20 minutes resulting in a slightly improved cell efficiency due to a marginal increase in short circuit current. The CdS/CIGS reference solar cell degrades in FF as well as short circuit current density which do not recover under light soaking leading to an efficiency drop (Table 1).
In conclusion, the wide bandgap and non-toxic material TiO2 deposited by ALD was successfully used as the n-type buffer layer for non-vacuum deposited CIGS thin film solar cells on flexible stainless steel substrates. A Jsc gain of 2.0 mA/cm2 was achieved by substitution of the conventional CdS buffer layer, resulting in a photocurrent of 38.9 mA/cm2. The ultrathin TiO2 layer dramatically enhanced the photocurrent gain in the UV spectrum without compromising Voc due to its homogeneous and conformal coverage, inherent to the ALD deposition process and possibly a surface inversion at the TiO2/CIGS interface. We conclude that the ultrathin amorphous TiO2 layer is a promising candidate for the application in high efficiency CIGS thin film solar cells to further boost their performance.
Methods
Cu(In,Ga)Se2 preparation
Metallic oxide powders of In2O3, Ga2O3 and Cu2O were mixed homogeneously with DI water and subsequently coated onto the Mo/Cr substrate by using a doctor blade. The precursor was converted to CIGS first by reduction in hydrogen (H2) replace: followed by selenization for 20 minutes in hydrogen selenide (5% H2Se in Ar), both at a temperature of 500 °C.
Solar cell device fabrication
The following device architecture was employed: stainless steel foil/Cr/Mo/CIGS/CdS or TiO2/ITO/Ag. 1000 nm Cr and 800 nm Mo were deposited on 75 μm stainless steel foil substrate by DC sputtering used as diffusion barrier and back contact, respectively. Before depositing the n-type buffer layer, the CIGS absorber layers were rinsed in 5 wt% potassium cyanide solution for 5 minutes to remove excess CuSex. Next, the CIGS absorbers were coated with TiO2 by atomic layer deposition (ALD). TiO2 thicknesses varied from 0–30 nm and deposition temperatures ranged from 110–180 °C. Titanium isopropoxide (Ti[OCH(CH3)2]4) and H2O were used as ALD precursors. The TiO2 deposition rate is about 0.25 Å/s measured by ellipsometry on test films grown on Si wafer substrates. A reference sample was immersed under optimized conditions24,25 in a chemical bath to deposit 50 nm CdS at a temperature of 70 °C (details can be found in ref. 24). 50 nm indium tin oxide (ITO) as transparent conductive layer was deposited on both CdS/CIGS and TiO2/CIGS devices by RF sputtering at room temperature (ITO target: In2O3:SnO2 = 90%:10%). ITO was deposited by a mild off-angle sputtering condition using 40 Watt and 0.9 mTorr Ar pressure with a sample-to-target distance of 17 cm. Finally, shadow masks were used to define the metal grid patterns. 100 nm thick Ag (thermally evaporated) grid with finger width and pitch of 100 μm and 800 μm, respectively, were deposited by thermal evaporation. Individual cells of 3.5 × 3.5 mm2 size were manually scribed resulting in an active area of 10.5 mm2.
Focused ion beam preparation
The sample was coated with Pt to prevent damage during the FIB cut. FIB was done on a FEI Quanta 3D FEG with Ga ions using 30 kV and 50 pA.
Scanning electron microscope
Cross sectional scanning electron micrographs were taken on a Zeiss Gemini Ultra-55.
Simulation
The 1D solar cell simulation software SCAPS22 was used to simulate the CIS/CdS/ITO and CIS/TiO2 /ITO interface. The band gaps and electron affinities of 0.96 eV and 4.6 eV (CIS), 3.4 eV and 4.0 eV (TiO2), 2.4 eV and 4.3 eV (CdS) and 3.3 eV and 4.4 eV (ITO) were used for the simulations.
Solar cell performance measurement: J-V, EQE and 1-R%
Solar cells were characterized under simulated 1-sun illumination (1000 W/m2, global air mass 1.5 spectrum, 25 °C). EQE was measured in a QE-R system from Enlitech using a 150W XQ lamp. Calibrated Si (300 nm–1100 nm) and a Ge (1100 nm–1800 nm) diodes were used as references for the EQE measurement. Barium sulfate was used as calibration sample to represent 100% reflectance.
Transmission and reflection measurements
Done with a Lambda 950 UV/VIS Spectrometer from PerkinElmer using a tungsten lamp. A% was extracted from reflectance R% and transmission T% spectroscopy via A% =100 % – R% – T%, where R% and T% are corrected for the quartz glass substrate.
Additional Information
How to cite this article: Hsu, W. et al. Electron-Selective TiO2 Contact for Cu(In,Ga)Se2 Solar Cells. Sci. Rep. 5, 16028; doi: 10.1038/srep16028 (2015).
References
Green, M. A., Emery, K., Hishikawa, Y., Warta, W. & Dunlop, E. D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 23, 1–9 (2015).
Jackson, P. et al. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Phys. Status Solidi RRL—Rapid Res. Lett. 9, 28–31 (2015).
Nakada, T., Mizutani, M., Hagiwara, Y. & Kunioka, A. High-efficiency Cu(In,Ga)Se2 thin-film solar cells with a CBD-ZnS buffer layer. Sol. Energy Mater. Sol. Cells 67, 255–260 (2001).
Solar Frontier Press Release, 2 April 2014. Solar frontier sets thin film PV world record with 20.9% CIS cell. Available at: http://www.solar-frontier.com/eng/news/2014/C031367.html. (accessed: 22nd July 2015).
Hultqvist, A., Platzer-Björkman, C., Törndahl, T., Ruth, M. & Edoff, M. Optimization of i-ZnO window layers for Cu(In,Ga)Se2 solar cells with ALD buffers. in Proceedings of the 22nd European Photovoltaic Solar Energy Conference 2381–2384 (2007).
Hariskos, D., Spiering, S. & Powalla, M. Buffer layers in Cu(In,Ga)Se2 solar cells and modules. Thin Solid Films 480–481, 99–109 (2005).
Witte, W., Spiering, S. & Hariskos, D. Substitution of the CdS buffer layer in CIGS thin-film solar cells. Vak. Forsch. Prax. 26, 23–27 (2014).
Vidal, J., Vigil, O., de Melo, O., López, N. & Zelaya-Angel, O. Influence of NH3 concentration and annealing in the properties of chemical bath deposited ZnS films. Mater. Chem. Phys. 61, 139–142 (1999).
Törndahl, T., Platzer-Björkman, C., Kessler, J. & Edoff, M. Atomic layer deposition of Zn1−xMgxO buffer layers for Cu(In,Ga)Se2 solar cells. Prog. Photovolt. Res. Appl. 15, 225–235 (2007).
Hariskos, D. et al. The Zn(S,O,OH)/ZnMgO buffer in thin-film Cu(In,Ga)(Se,S)2-based solar cells part II: Magnetron sputtering of the ZnMgO buffer layer for in-line co-evaporated Cu(In,Ga)Se2 solar cells. Prog. Photovolt. Res. Appl. 17, 479–488 (2009).
ZSW press release, ZSW raises efficiency of cadmium-free CIGS solar cells to record 21%. Available at: http://www.semiconductor-today.com/news_items/2015/feb/zsw_250215.shtml. (Accessed: 17th July 2015).
Hubert, C. et al. A better understanding of the growth mechanism of Zn(S,O,OH) chemical bath deposited buffer layers for high efficiency Cu(In,Ga)(S,Se)2 solar cells. Phys. Status Solidi A 205, 2335–2339 (2008).
Shin, D. H. et al. Improvement of the cell performance in the ZnS/Cu(In,Ga)Se2 solar cells by the sputter deposition of a bilayer ZnO : Al film. Prog. Photovolt. Res. Appl. 21, 217–225 (2013).
Kushiya, K. Development of Cu(InGa)Se2-based thin-film PV modules with a Zn(O,S,OH)x buffer layer. Sol. Energy 77, 717–724 (2004).
Kobayashi, T., Yamaguchi, H. & Nakada, T. Effects of combined heat and light soaking on device performance of Cu(In,Ga)Se2 solar cells with ZnS(O,OH) buffer layer. Prog. Photovolt. Res. Appl. 22, 115–121 (2014).
Chirilă, A. et al. Highly efficient Cu(In,Ga)Se2 solar cells grown on flexible polymer films. Nat. Mater. 10, 857–861 (2011).
Jackson, P. et al. High quality baseline for high efficiency, Cu(In1−x,Gax)Se2 solar cells. Prog. Photovolt. Res. Appl. 15, 507–519 (2007).
Brown, G., Stone, P., Woodruff, J., Cardozo, B. & Jackrel, D. Device characteristics of a 17.1% efficient solar cell deposited by a non-vacuum printing method on flexible foil. in 2012 38th IEEE Photovoltaic Specialists Conference (PVSC) 003230–003233 (2012).
Johnson, R. W., Hultqvist, A. & Bent, S. F. A brief review of atomic layer deposition: from fundamentals to applications. Mater. Today 17, 236–246 (2014).
Bär, M. et al. Electronic level alignment at the deeply buried absorber/Mo interface in chalcopyrite-based thin film solar cells. Appl. Phys. Lett. 93, 042110 (2008).
Yin, X. et al. 19.2% Efficient InP Heterojunction Solar Cell with Electron-Selective TiO2 Contact. ACS Photonics 1, 1245–1250 (2014).
Burgelman, M., Nollet, P. & Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Films 361–362, 527–532 (2000).
Repins, I. et al. Kesterites and Chalcopyrites: A Comparison of Close Cousins. in Symposium D—Compound Semiconductors for Energy Applications and Environmental Sustainability 1324 (2011).
Hsu, W.-T., Ro, S.-S., Hsu, H.-R. & Liu, Y.-C. N-type CdS layer prepared by shallow chemical bath deposition on a 370 × 470 mm2 glass substrate. Thin Solid Films 529, 253–256 (2013).
Contreras, M. A. et al. Optimization of CBD CdS process in high-efficiency Cu(In,Ga)Se2-based solar cells. Thin Solid Films 403–404, 204–211 (2002).
Acknowledgements
W.H. acknowledges funding from ITRI. TiO2/CIGS solar cell fabrication and characterization was funded by the Bay Area Photovoltaics Consortium (BAPVC). Materials characterization part of this work was supported by the Director, Office of Science, Office of Basic Energy Sciences, Material Sciences and Engineering Division of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
W.H., M.H., C.-C.C. and A.J. designed the experiments. W.H., M.H., C.M.S.-F., L.C., S.C., Y.C., Y.Z., M.Z. and H.-P.W. carried out the experiments. W.H., M.H., C.M.S.-F., M.Z., H.-P.W. and A.J. contributed to the data analysis. W.H., C.M.S.-F. and A.J. wrote the paper while all authors provided feedback.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hsu, W., Sutter-Fella, C., Hettick, M. et al. Electron-Selective TiO2 Contact for Cu(In,Ga)Se2 Solar Cells. Sci Rep 5, 16028 (2015). https://doi.org/10.1038/srep16028
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16028
This article is cited by
-
Investigation of TiO2 as the buffer layer in wide bandgap chalcopyrite solar cells using SCAPS
Journal of Materials Science: Materials in Electronics (2022)
-
Thin oxide buffer layers for avoiding leaks in CIGS solar cells; a theoretical analysis
Journal of Materials Science: Materials in Electronics (2021)
-
Progress and prospects for ultrathin solar cells
Nature Energy (2020)
-
Mechanochemical Solvent-Free Synthesis of Quaternary Semiconductor Cu-Fe-Sn-S Nanocrystals
Nanoscale Research Letters (2017)
-
Chalcogenide mechanochemistry in materials science: insight into synthesis and applications (a review)
Journal of Materials Science (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.