Abstract
Introduction:
Extramedullary hematopoiesis (EMH) is the production of blood cell precursors outside the bone marrow that occur in various hematological diseases. In patients with thalassemia intermedia, ineffective erythropoiesis drives compensatory EMH in the liver, pancreas, pleura, spleen, ribs and spine.
Case Presentation:
We describe a patient with thalassemia intermedia who presented with acute neurological symptoms caused by paraspinal EMH, which responded well to combination therapy of steroid, hypertransfusion, laminectomy and excision of pseudotumor and hydroxyurea therapy to boost the formation of fetal haemoglobin.
Discussion:
Prompt recognition of EMH based on clinical presentation and typical radiological findings should be made. Early treatment is recommended to prevent irreversible damage to the spinal cord.
Similar content being viewed by others
Introduction
Thalassemia is the most common single gene disorder in Malaysia. The clinical phenotypes of thalassemia intermedia lie between those of thalassemia minor and major.1 These patients require occasional or no transfusions as they can usually maintain hemoglobin levels between 7 and 10 g dl−1, and transfusions can lead to iron overload, necessitating chelation therapy.1 Ineffective erythropoiesis in patients with thalassemia intermedia drives compensatory extramedullary hematopoiesis (EMH) in the liver, pancreas, pleura, spleen, ribs and spine.1 EMH is the production of blood cell precursors outside the bone marrow that occur in various disorders such as thalassemia, sickle cell anemia, hereditary spherocytosis, polycythemia vera, myelofibrosis and other hematological diseases.2
Paraspinal EMH deserves a special attention as it may cause compression of the spinal cord, which leads to debilitating symptoms and permanent disability if left untreated. Thus early recognition and subsequent treatment are important to prevent permanent damage to the spinal cord.
Case presentation
We describe a 26-year-old Chinese man who was diagnosed with thalassemia intermedia at the age of 12 when he presented with symptoms of cholelithiatis. Open cholecystectomy was done at that time. Subsequently, he required one or two blood transfusions every year, but otherwise he was well.
He presented to us 14 years later complaining of bilateral lower limb paresthesia, and 4 days after that he suddenly had bilateral lower limb weakness, which led to presentation to the hospital. He was still able to walk, but the gait was unsteady. Otherwise, he had no symptoms of the upper limbs, bladder or bowel. He had no history of fever or trauma prior to the symptoms. However, he was discharged on the same day after the investigation, which revealed an acceptable hemoglobin level of 8.5 g dl−1 with mean cell volume of 64 fl (normal 77−97 fl) and mean cell hemoglobin of 19.5 pg (normal 27.0−32.0 pg). His peripheral blood film showed hypochromic microcytic anemia with anisocytosis, increased target cells, polychromatic cells, tear drop cells, bite cells, blister cells and schistocytes. There was no nucleated red blood cell seen. The red cells' morphology was consistent with thalassemia.
He came back 3 days later as the symptoms were worsening. The weakness caused him to hold onto the wall or furniture to balance himself when he was walking. He also had difficulty in passing urine by that time.
Physical examination revealed a thin young man with pallor and a tinge of jaundice. The apex beat was displaced with systolic ejection murmur at the left sternal edge. There was hepatosplenomegaly with evidence of cholecystectomy scar in the right hypochondriac region. Neurological examination of the upper limbs was normal. Lower limb examination revealed increased tone bilaterally with nonsustained clonus of both ankles. Muscle power was 3/5 over the hips, 4/5 over both knees and 4/5 over the ankles. The ankle and knee jerks were hyperreflexic bilaterally. Sensation to pin prick was intact till T4 dermatome and impaired from T5 dermatome downward. Plantar responses were upgoing bilaterally. Rectal examination revealed the presence of deep anal sensation and voluntary anal contraction. Bulbocavernosus reflex was present.
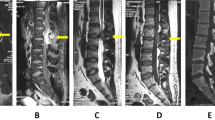
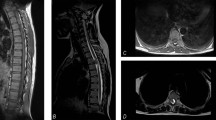
His hemoglobin was 7.4 g dl−1 at that time. Chest X-ray showed widened right paraspinal stripe with lobulation likely to represent posterior mediastinal mass (Figure 1). The spine magnetic resonance imaging (MRI) (Figures 2,3,4) revealed extension of paraspinal EMH into the extradural space causing spinal cord compression at T3–T9 levels. The spinal canal is narrowest at the T8 level measuring 4 mm in AP diameter. The left T5 and right T6 exiting nerve roots are obliterated. No spinal cord signal changes are seen.
He was started on dexamethasone to shrink the tumors and had three units packed red blood cell transfusions preoperatively to maintain the hemoglobin at 10g dl−1 to decrease proliferative stimulus as well as to optimize his hemoglobin in anticipation of operative blood lost. He had an uneventful laminectomy of T5–T8 with excision of tumor. He had another two units of packed red blood cell transfusion intraoperatively. The removed tumor was soft to firm in consistency with an appearance similar to blood clot. It was firmly adherent to the removed laminae. His motor power improved after the operation with a lower limb myotome power of 5/5 except for the L5 myotome with a power of 4/5. However, the sensory impairment remained the same. The bladder function returned to normal and bowel function remained normal. He was referred for inpatient rehabilitation and was discharged 6 days after that, walking safely with the aid of a walking frame. He was prescribed oral Hydroxyurea 500 mg daily to increase the fetal haemoglobin and reduce the proliferative stimulus. A few months after that, he was able to walk unaided. The histopathological reports confirmed the removed tumor as EMH.
Discussion
Differential diagnoses for compression from extramedullary mass include neurogenic tumor, lymphoma, metastases, lateral meningocele, extrapleural cyst and epidural abscess.3,4 Although biopsy is the gold standard for tissue diagnosis, it is invasive and carries the risk of catastrophic hemorrhage.5 MRI has become the method of choice for the diagnosis and follow-up evaluation for spinal cord compression from EMH.6
As paraspinal EMH is a rare condition, evidence-based therapy from randomized control trials is lacking.7,8 Most of the reported treatment options are based on case reports and small case series.8 Management options include hypertransfusion, radiotherapy, surgical decompression, hydroxyurea or a combination of these modalities. Treatment should be individualized as each treatment option carries its own advantages and disadvantages.9 Our patient had a combination of steroid, hypertransfusion, surgical decompression and hydroxyurea.
Hypertransfusion means to maintain the hemoglobin between 9.0 and 10.5 g dl−1. As EMH is a compensatory mechanism for chronic anemia, correction by recurrent blood transfusion can cause these tumors to shrink; thus the spinal cord compression is alleviated, which leads to clinical neurologic improvements. Hypertransfusion can also be used as a diagnostic tool as only cases of spinal cord compression from EMH would respond to transfusion. However, there is a risk of transfusion reaction, infection, iron overload and alloimmunization.10 In addition, a few studies demonstrated that the improvement is usually slow, insufficient and temporary.11 Thus we used blood transfusion as an adjunct to surgical decompression to reduce the size and to prevent recurrence in view of operative blood loss.
Surgical decompression is indicated in acute cases that do not respond to adequate transfusion or radiotherapy.7 Sbeih et al. suggested surgical decompression for patients with power less than 3/5 and not ambulatory, or for patients with progressive rapid loss in neurological function or sphincteric impairment,11 like in this case. Surgery grants the benefits of immediate relief from cord compression and provides histological diagnosis.10 Diagnostic needle biopsies are not recommended as they carry the risk of bleeding.5 The drawbacks to surgery include the risk of bleeding due to high vascularity of the tumor and the risk of operating on anemic individuals, which can lead to shock as a result of operative blood loss.10,12 There are also risks of infection, lower limb paralysis, bladder and bowel problem as well as cerebrospinal fluid leakage. Inadequate excision is a possibility in patients with diffuse EMH. Spine instability and kyphosis can occur as a result of multilevel laminectomies.12 Immediate total resection of EMH can cause clinical deterioration and compensation by more EMH, as these masses play an important role in maintaining an adequate hemoglobin level.
Hematopoietic tissue is extremely radiosensitive, making radiotherapy an option for EMH, either alone or in combination with other therapies. However, there is a high risk of recurrence and radiotoxicity on an already compromised spinal cord.10 Tissue edema and pancytopenia resulting from this therapy might worsen the neurological symptoms. Redda et al.8 reported no acute toxicity using total radiotherapy dose of 10 Gy in five sessions of 2 Gy per day. They also reported significant pain relief after first radiotherapy fraction. Radiotherapy is commonly used as an adjunct post decompression to reduce recurrence.
Hydroxyurea, a ribonucleotide reductase inhibitor, can be used to stimulate synthesis of fetal hemoglobin. It is suitable as a monotherapy for patients who are contraindicated for transfusion or as a combination therapy. Redda et al. observed improvement in hematological parameters after 6 months of hydroxyurea therapy.8
In conclusion, a prompt recognition of the condition based on clinical presentation and typical radiological findings should be made. Early treatment is recommended to prevent irreversible damage to the spinal cord.
References
Taher A, Isma'eel H, Cappellini MD . Thalassemia intermedia: revisited. Blood Cells Mol Dis 2006; 37: 12–20.
Soman S, Rosenfeld DL, Roychowdhury S, Drachtman RA, Cohler A . Cord compression due to extramedullary hematopoiesis in an adolescent with known beta thalassemia major. J Radiol Case Rep 2009; 3: 17–22.
Savini P, Lanzi A, Marano G, Moretti CC, Poletti G, Musardo G et al. Paraparesis induced by extramedullary haematopoiesis. World J Radiol 2011; 3: 82–84.
Dibbern DAJr, Loevner LA, Lieberman AP, Salhany KE, Freese A, Marcotte PJ . MR of thoracic cord compression caused by epidural extramedullary hematopoiesis in myelodysplastic syndrome. AJNR Am J Neuroradiol 1997; 18 (2):363–366.
Ibrahim AW, Ibrahim EM, Mitry NM, Abdul-Satir A, Kuppa A . Spinal cord compression due to intrathoracic extramedullary haematopoiesis in homozygous thalassaemia. J Neurol Neurosurg Psychiatry 1983; 46 (8):780–782.
Cos¸kun E, Keskin A, Süzer T, Sermez Y, Kıldacı T, Tahta K . Spinal cord compression secondary to extramedullary hematopoiesis in thalassemia intermedia. Eur Spine J 1998; 7: 501–504.
Haidar R, Mhaidli H, Taher AT . Paraspinal extramedullary hematopoiesis in patients with thalassemia intermedia. Eur Spine J 2010; 19: 871–878.
Ruo Redda MG, Allis S, Reali A, Bartoncini S, Roggero S, Anglesio SM et al. Complete recovery from paraparesis in spinal cord compression due to extramedullary haemopoiesis in beta-thalassaemia by emergency radiation therapy. Intern Med J 2014; 44: 409–412.
Moncef B, Hafedh J . Management of spinal cord compression caused by extramedullary hematopoiesis in beta-thalassemia. Intern Med 2008; 47: 1125–1128.
Salehi SA, Koski T, Ondra SL . Spinal cord compression in beta-thalassemia: case report and review of the literature. Spinal Cord 2004; 42: 117–123.
Sbeih IAA, Abdelal OM, Ghanem MHE, Ayyad A . Spinal cord compression by extramedullary haematopoietic tissue in a patient with β-thalassaemia. Pan Arab J Neurosur 2008; 12: 80–87.
Malik M, Pillai LS, Gogia N, Puri T, Mahapatra M, Sharma DN et al. Paraplegia due to extramedullary hematopoiesis in thalassemia treated successfully with radiation therapy. Haematologica 2007; 92: e28–e30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hisamud-din, N., Mustafah, N., Fauzi, A. et al. Incomplete paraplegia caused by extramedullary hematopoiesis in a patient with thalassemia intermedia. Spinal Cord Ser Cases 3, 17020 (2017). https://doi.org/10.1038/scsandc.2017.20
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.20
This article is cited by
-
Extramedullary hematopoiesis in β-thalassemia major patient: a case report and review of the literature
Journal of Hematopathology (2022)