Abstract
Study design:
Randomized crossover.
Objectives:
To analyze the acute effects of isolated and simultaneous application of whole-body vibration (WBV) and electromyostimulation (ES) on popliteal artery blood velocity (BV) and skin temperature (ST) of the calf in subjects with spinal cord injury (SCI).
Setting:
Valladolid, Spain.
Methods:
Ten subjects with SCI were assessed in five different sessions. After a familiarization session, four interventions were applied in random order; WBV, ES, simultaneous WBV and ES (WBV+ES), and 30 s of WBV followed by 30 s of ES (WBV30/ES30). Each intervention consisted of 10 sets × 1 min ON+1 min OFF. Subjects were seated on their own wheelchairs with their feet on the vibration platform (10 Hz, 5 mm peak-to-peak), and ES was applied on the gastrocnemius muscle of both legs (8 Hz, 400 μs).
Results:
The simultaneous application (WBV+ES) produced the greatest increase in mean BV (MBV; 36% and 42%, respectively) and peak BV (PBV; 30% and 36%, respectively) during the intervention. This intervention produced the greatest mean increases in MBV (21%) and PBV (19%) during the recovery period. Last, this intervention produced the highest increase in ST during the intervention (2.1 °C).
Conclusion:
The simultaneous application of WBV+ES seems to produce a greater increase in MBV and PBV of the popliteal artery and ST of the calf than the isolated (WBV or ES) or consecutive application of both stimuli (WBV30/ES30). This study provides an efficient therapeutic methodology to improve peripheral arterial properties, which is pivotal in SCI patient’s rehabilitation.
Similar content being viewed by others
Introduction
Spinal Cord Injury (SCI) is one of the most dramatic clinical conditions due to the loss of voluntary muscle contraction that leads to physical disability. SCI patients exibit an increased risk of vascular diseases, due to an extremely low physical activity,1 when compared with counterparts without SCI.2 Accordingly, earlier cardiovascular disease is the leading cause of death in SCI patients.2 However, common cardiovascular risk factors (for example, sex, age, diabetes, blood lipid profile, elevated systolic blood pressure and smoking status3) do not seem to fully explain the increased cardiovascular risk in SCI patients.4 Recent studies have focused on peripheral vascular factors as an underlying mechanism for the increased cardiovascular risk.5 Moreover, adverse peripheral vascular adaptations appear to be a plausible mechanism for the development of pressure ulcers due to the significant reduction in lower limb arterial blood flow.6 Pressure ulcers is one of the most common complications and leading cause of re-hospitalization in SCI patients.7
Structural and functional adaptations have been detected in peripheral arteries below the level of injury almost immediately after SCI.8, 9 Indeed, a 30% reduction in the common femoral artery diameter and resting leg blood flow occurs within the first 6 weeks of inactivity and paralysis.8 Moreover, a 50 and 40% decrease in the common femoral artery diameter and resting leg blood flow are evident beyond this early stage.5 These early vascular adaptations seem to be closely related to the decrease in metabolic demand after SCI.10 In response to a vasodilatory stimulus, absolute peak blood flow (PBF) reactivity to thigh muscle ischemia was reduced in SCI patients; however, after adjusting the change in PBF for the reduced muscle volume eliminated between-group differences, suggesting that arterial dysfunction and muscle atrophy occur simultaneously. In addition, other mechanisms that appear to contribute to this arterial dysfunction include inhibition of the muscle pump as a result of the inability to contract the skeletal muscles11 and the consequent reduction in the arterio-venous pressure gradient across the muscle bed.12
Muscle contraction provide a powerful stimulus for vascular remodeling.13 Angiogenesis14 and increased arterial diameter15 seem to be the principal peripheral vascular adaptations induced by exercise training. Passive mobilization, the most basic form of rehabilitation for SCI patients, has failed to revert arterial dysfunction.11 However, there are other rehabilitation tools that have shown effectiveness in SCI. Electromyoestimulation (ES) involves the application of electrical stimuli to superficial skeletal muscles, with the main objective of triggering visible and valid muscle contractions through activation of motorneurons or intramuscular axonal branches.16 The effect of ES on peripheral blood flow is intensity dependent. Although, ES can increase local blood flow,17 excessive intensity might lead to partial ischemia; whereas, insufficient intensity might be inadequate to significantly increase arterial blood flow.18 Accordingly, a single bout of ES has been shown to improve leg blood flow in SCI patients.19
A new rehabilitation tool is whole-body vibration (WBV), which refers to mechanical oscillations transferred to the skeletal muscles through a supporting system such as a seat or platform.20 The application of the vibration to the muscle-tendon complex produces a stretch-shortening action that activates muscle spindles and triggers the reflexive muscle contraction.21 To date, only three studies have investigated the acute effects of WBV on the cardiovascular system in individuals with paralysis. In the first study, 3–6 min of WBV elicited increases in oxygen consumption and oxygenation in the gastrocnemius muscle, suggesting increased perfusion.22 In the second study, the vibratory stimulus applied to the feet produced increases in femoral blood velocity in SCI patients.23 Moreover, Koutnik et al.24 observed that a 10-min set of passive vibration induced a favorable local vascular effect as shown by reduced leg arterial stiffness in post-stroke patients, reflecting improved peripheral vasodilation.
ES and WBV are effective methods to increase lower extremity blood flow. In a previous study by our research group, the simultaneous application of both methods (WBV+ES) produced a greater increase in mean blood velocity (MBV) and peak blood velocity (PBV) of the popliteal artery and skin temperature (ST) of the calf than the isolated or consecutive application of both stimuli in able-bodied young healthy males.25 However, to our knowledge, no study has evaluated the local vascular effect of the simultaneous or consecutive application of both stimuli in SCI patients. Therefore, the aim of this study was to examine the acute effects of isolated, consecutive, and simultaneous application of WBV and ES on the popliteal arterial blood velocity and ST of the calf in SCI patients. We hypothesized that WBV+ES would elicit greater blood velocities and ST responses than the isolated or consecutive interventions in SCI patients.
Materials and methods
Participants
Ten patients (eight males and two females) with SCI volunteered to participate in the study. All the patients used wheelchair for their locomotion. All participants were classified by the American Spinal Injury Association (ASIA) as A or B. None of the subjects had any history of disease such as peripheral arterial disease or other confounding factors. Medications were recorded and only antispasticity drugs were allowed during the study. All participants received 10 2-h rehabilitation sessions per month, which consisted of standing (tilted) position, passive movements, low-intensity resistance training or electrotherapy and physiotherapy treatment. Experimental interventions were applied to the subjects before their rehabilitation routines. Each participant gave written informed consent to participate in the investigation with the risks and benefits of the study carefully explained to them before their initiation. The study was conducted according to the Declaration of Helsinki and was approved by the University Committee on Human Research. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Experimental design
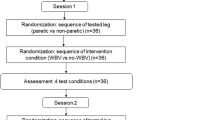
Each participant was assessed in five different sessions separated by at least 48 h. Participants completed all sessions at the same time of day to avoid variations in arterial function. In the first session, participants were familiarized with the testing procedures and interventions. In the remaining four sessions, the four interventions were applied in random order. The four interventions were: WBV alone (WBV), ES alone (ES), simultaneous WBV and ES (WBV+ES), and 30 s of WBV followed by 30 s of ES (WBV30/ES30). Each session consisted of 10 sets of 1 min of intervention and 1 min of rest between sets. Participants were seated on their own wheelchair with feet placed on the vibration platform (Galileo Home, Galileo, Novotec, Germany). Measurements were collected before and for 5 min after the intervention (R1–R5; Figure 1).
Methods
Participants rested in the seated position for a period of 10 min to normalize his blood flow before data collection and the application of the corresponding intervention. During all interventions, participants wore the same athletic shoes to standardize for potential dampening of the vibration due to variability in the footwear.26 The frequency of vibration was set at 10 Hz and the amplitude at 5 mm (peak-to-peak). The vertical component of acceleration was measured by an accelerometer (VT-6360, Hong Kong, China). The acceleration (peak) was 66.70 m s−2. Feet were placed parallel to each other 38 cm apart (measured from the midline of the heels). When ES was applied, a rectangular, biphasic and symmetric wave with a pulse width of 400 μs and 8 Hz was applied (Compex 3, DJO Ibérica, Madrid, Spain). The muscle was stimulated indirectly via the motor axon terminal branches. Three, 2-mm-thick, self-adhesive electrodes were used on each leg: one electrode (10 × 5 cm) was placed 2 cm below the popliteal fold and two electrodes (5 × 5 cm) were placed as close as possible to the motor points of the gastrocnemius medialis and lateralis. Current intensity was increased until the subject’s motor threshold (mean achieved intensity: 46.6±12.3 mA; min/max; 30/61 mA). This intensity, reached in the first treatment session, was applied in the following sessions using ES.
Measurements
Doppler velocity waveforms were registered at baseline, during the resting minute (in the final 30 s) between the 10 sets, and at the end of each minute during the recovery period (Figure 1). The popliteal artery was imaged in a longitudinal section with an ultrasound system (MyLab 25, Esaote, Genoa, Italy) using a pulsed color Doppler with a linear array transducer (LA 523, 7.5–12 MHz; length, 50 mm; Esaote) on the right popliteal fossa. The probe was positioned to maintain an insonation angle ⩽60°. Each ultrasound image was recorded for a period of 4 s. In that period, there were between 3 and 5 beats, and the mean of these beats was used for data analyses (MyLab Desk 8.0, Esaote) to obtain mean (MBV) and peak (PBV) systolic blood velocities (Figure 2). Before the blood flow parameters were collected, ST over the left gastrocnemius was measured using an infrared thermometer (PCE-777, PCE Instruments, Southampton, UK). In order to collect a consistent measurement, an ink mark was made between the two gastrocnemius heads. Participants were asked to redo this mark at home to ensure reliability of the measurements throughout all the sessions. All measurements were performed by the same researcher.
Data analyses
The normality of the dependent variables was checked and subsequently confirmed using the Shapiro–Wilk test. A two-way repeated measures analysis of variance in treatment and time was applied. When a significant F-value was achieved, pairwise comparisons were performed using the Bonferroni post hoc procedure. Effect size statistic, η2, was provided to determine the magnitude of the effect independently of the sample size. Statistical significance was set at P⩽0.05. Values are expressed as mean±s.d. The percentage of variation in respect to baseline values was calculated as: (value−baseline) × 100/baseline.
Results
Table 1 summarizes the characteristics of the participants.
Mean blood velocity
A time × intervention effect was observed for the MBV (F=1.755; P<0.01; η2=0.163). The percentage of variation compared with baseline and the statistical differences are shown in Figure 2. For the WBV intervention, MBV was increased from T1 (13.1% P<0.05) to R2 (11.2% P<0.01) compared with baseline. For the ES intervention, MBV increment compared with baseline was observed from T8 (7.1% P<0.001) to T10 (8.9% P<0.05). The WBV+ES intervention remained elevated in the MBV from T1 (22.1% P<0.001) to R3 (18.7% P<0.05). Finally, for the WBV30/ES30 intervention, MBV increase compared with baseline was observed from T1 (18.6% P<0.05) to R1 (23.7% P<0.05).
The peak increments during the application of each intervention were 27±4%, 13±4.9%, 42±3.9% and 30±4.8% for WBV, ES, WBV+ES and WBV30/ES30, respectively; the mean increments during the application of each intervention were 22±2.2%, 10±2.5%, 36±1.3% and 27±2% for WBV, ES, WBV+ES and WBV30/ES30, respectively; and the mean increments during the resting period of each intervention were 10±2.1%, 1±2.5%, 21±3% and 13±3.7% for WBV, ES, WBV+ES and WBV30/ES30, respectively.
Peak blood velocity
A time × intervention effect was observed in the PBV (F=1.542; P<0.05; η2=0.146). The percentage of variation compared with baseline and the statistical differences are shown in Figure 3. For the WBV intervention, PBV was increased from T1 (11.3% P<0.05) to R2 (11.4% P<0.05) compared with baseline. For the ES intervention, PBV increased compared with baseline at T7 (15.5% P<0.05) to T9 (9.8% P<0.05). The WBV+ES intervention elevated the PBV above baseline during all the intervention from T1 (20.5% P<0.001) to R5 (16.4% P<0.05). Finally, for the WBV30/ES30 intervention, PBV was increased compared with baseline from T1 (15.3% P<0.05) to R1 (22.6% P<0.05).
The peak increments during the application of each intervention were 24±6.6%, 17±7.3%, 36±4.4% and 28±13.6% for WBV, ES, WBV+ES and WBV30/ES30, respectively. The mean increments during the application of each intervention were 19±3.2%, 13±4.8%, 30±2.5% and 21±4.6% for WBV, ES, WBV+ES and WBV30/ES30, respectively. The mean increments during the resting period of each intervention were 11±4.8, 2±4.9, 19±4.7 and 11±7.6% for WBV, ES, WBV+ES and WBV30/ES30, respectively.
Skin temperature
A time × intervention effect was also observed for ST (F=1.974; P<0.001; η2=0.180). The absolute values and the statistical differences are shown in Figure 4. The simultaneous intervention (WBV+ES) was the intervention that increased ST above baseline (24.5 °C) earlier than the WBV, from T4 (29.6 °C P<0.05) to R5 (30.3 °C P<0.05). The WBV intervention increased ST compared with baseline (25.5 °C) from T9 (29.8 °C P<0.05) to R5 (29.9 °C P<0.05). For the ES and the WBV30/ES30 interventions, ST did not increase compared with baseline.
The peak increments during the application of each intervention were 1.5±0.7, 1±0.5, 2.1±0.4 and 1.4±0.61.8 °C for WBV, ES, WBV+ES and WBV30/ES30, respectively.
Discussion
To our knowledge, this is the first study that has evaluated the simultaneous application of WBV and ES in SCI patients. The major finding of this study was that the four interventions enhanced MBV and PBV in respect to baseline values. The simultaneous application of both stimuli (WBV+ES) seems to produce the greatest mean and peak increase in MBV (36% and 42%, respectively) and PBV (30% and 36%, respectively) during the intervention. Importantly, this intervention produced the greatest mean increases in MBV (21%) and PBV (19%) during the recovery period. Similarly, WBV+ES maintained MBV above baseline for longer time than the isolated (WBV or ES) or consecutive application of both stimuli (WBV30/ES30), from T1 (22.1%) to R3 (18.7%). Moreover, WBV+ES was the only intervention that maintained PBV above baseline during all the protocol; from T1 (20.5%) to R5 (16.4%). Lastly, WBV+ES produced the earliest and highest increase in ST.
The WBV intervention produced an increase in MBV and PBV in the popliteal artery. The only previous study that had examined the acute effect of WBV on arterial blood flow in people with SCI showed the ability of the vibration therapy to produce an acute increase in MBV and PBV.23 Although only 3 min of WBV was applied, the magnitude of the increase in MBV (36%) at the end of the third minute in the previous study was higher than the increase observed at the third minute (21.5%) of the isolated application of WBV in the present study. The most likely explanation for this discrepancy could be the different mechanical load on the platform between both studies. In the previous study, patients were on a tilt table at 45°, whereas in the present study, patients were seated on their wheelchairs. It is likely that participants in the tilted position supported more body weight on the vibrating platform than those in the seated position resulting in more intense reflexive contractions. Future studies should investigate vibration transmissibility with the feet on a vibrating platform while seated. The magnitude of the increase in muscle perfusion associated with WBV appeared to be related to the vibratory load used.27 Moreover, differences in duration of vibration may influence vascular responses28 with greater vasodilatory effect after continuous than intermittent 3 min of vibration exposure. Evidence has shown that continuous 5–10 min of passive vibration can maintain local vasodilation and improve arterial function in the exposed limb for 5–15 min post vibration in older adults with type 2 diabetes29 and post stroke,24 respectively. Both reflexive and voluntary muscle contractions are often cited as the mechanisms responsible for active vibration-induced increases in muscle perfusion in healthy young men.30 In the absence of voluntary contractions, increased neuromuscular activity of the quadriceps muscle and local artery vasodilation have been demonstrated in response to passive vibration in SCI patients.23 These reflex contractions produce an increase in local metabolites that relax vascular smooth muscle and trigger an increase in blood flow via local vasodilatation.21 Otherwise, previous studies have observed an increment in nitric oxide (NO), a potent endothelial vasodilator, as a result of 5 min of passive vibration at 50 Hz due to the shear stress generated by the vibration.29 Therefore, our findings suggest that WBV causes a marked increase in popliteal artery blood velocity through metabolic and endothelial-mediated vasodilation.
Likewise, the ES intervention produced an increase in MBV and PBV of the popliteal artery. These results are in agreement with previous studies that observed an increase in femoral artery blood flow due to application of ES in SCI patients.19, 29 The natural calf muscle pump is activated during an ES intervention due to involuntary muscular contraction caused by the electric current. However, the ES protocol showed lower efficacy than the WBV intervention. During ES, activation of the motor units occurs primarily under the electrodes placement. This mechanism is particular and contrasts with the recruitment of muscle fibers induced during a voluntary contraction.17 Recruitment of mainly superficial muscle fibers by ES could contrast with activation of deeper muscle fibers by WBV. However, this pattern seems to be in opposition to what was observed during the application in healthy subjects, where the ES intervention showed a greater enhancement of MBV and PBV.25 In one hand, the intensity of ES was set at the motor threshold in SCI subjects. This intensity (46.6±12.3 mA) was similar to that achieved in healthy subjects using subject’s pain threshold (47.4±11.2 mA). The same intensity produced a lower response probably due to muscle deconditioning and atrophy. Thus, a higher intensity could be necessary in order to increase treatment effectiveness in SCI patients. However, further investigation is needed to understand the dose–response relationship between ES and vascular reactivity and the implication of sensory alterations in the adjustment of ES intensity. On the other hand, due to the particular pattern of muscular recruitment during ES, fatigue of the stimulated superficial muscle fibers may adversely affect muscle strength during an ES session.31 This ‘accommodation to the current’ is very common in ES training, and we believe it is quite likely the explanation for the lower effect of ES on MBV and PBV.25 This effect could take dramatic importance in those with SCI as they have more fatigable muscle fibers.19 In contrast, MBV and PBV values seem to be higher throughout the second half of the WBV intervention.
The greater efficacy observed with the simultaneous intervention compared with the isolated applications, is in consonance with the results from a previous study in healthy subjects.25 In young men, the vibratory stimulus offset the decline in popliteal artery blood velocity that occurred with isolated ES. Likewise, as hypothesized, the magnitude of the response was lower in SCI subjects (a MBV peak increment of 161% in healthy versus 42% in SCI subjects). The main explanation seems to be related to the vascular adaptations previously described in SCI subjects. These patients have shown a reduced vasodilatory capacity below the level of injury.16 In addition, previous studies have shown a decreased blood flow response in SCI with respect to healthy subjects due to the parallel vascular dysfunction and muscle atrophy.32 Reduced femoral artery dilatory capacity in SCI patients mainly reflects impaired NO-dependent vasodilation.33 In addition, the positive relationship between decreased leg artery diameter and lean mass in SCI patients34 suggests that increased local vascular resistance and reduced production of muscle metabolites may contribute to the impaired vasodilation in this population. Another factor could be that SCI patients were seated with the feet on the platform rather than in an active squat position, as is performed in healthy individuals. Therefore, SCI patients had reduced mechanical load. Nevertheless, the present study is the first to show an additive effect of WBV and ES in leg artery dilatory capacity, which may be explained by an enhanced production of muscle vasodilatory metabolites due to both stimuli and endothelial-NO production induced by WBV.29 Therefore, the combined therapy would be of great clinical interest for this population.
The simultaneous application produced an earlier and higher increase in ST as this effect was observed after the fourth set and persisted elevated during the recovery. Although WBV alone increased ST after the ninth set, the absence of a hyperthermic response in the calf throughout both ES interventions suggests that more intense and generalized muscle contractions are required to increase ST. The observed results are similar to those found in healthy men. In both studies, simultaneous therapy (WBV+ES) proved to be more effective to increase ST in the calf. This result is in consonance with other studies in which the vibration stimulus alone (50 Hz, 5–6 mm) was not sufficient to increase the ST after 10 min of application in healthy individuals.20, 35 Interestingly, despite no change in ST, a 10-min bout of WBV (26 Hz – 6 mm) increased muscle temperature by 1.5 °C in young adults.36 However, in previous studies,35, 37 vibration applied directly to the skin surface of the resting extremity (passive vibration) was effective to increase skin blood flow and ST against standing on the platform (active vibration). Of note, a large area of the extremity directly exposed to passive vibration may account for the local increase in ST. The results of this study suggest the possibility that the ST may reflect an increase in local superficial blood flow. Increased skin blood flow during passive vibration may be attributed to cell friction37 and not to increased energy metabolism to sustain muscle contraction. Increased skin blood flow is crucial for the wound-healing process20 in order to prevent and minimize the development of pressure ulcers. Thus, the simultaneous application of WBV and ES represent a highly practical intervention in subjects with SCI.
Finally, when both WBV and ES were applied within the same intervention, the consecutive application (WBV30+ES30) seems to evoke a lower response than the concomitant application (WBV+ES). This difference could be due to the recruitment of different muscle fibers by the combined stimulus as it has been previously explained. Indeed, the addition of the peak MBV and PBV after ther fifth set of WBV and ES alone are close to the peak value induced by WBV+ES. Therefore, our findings support the theory of an ‘additive vasodilatory effect’ as it was previously suggested.25
In conclusion, the simultaneous application of WBV and ES seems to produce a greater increase in MBV and PBV of the popliteal artery and ST of the calf than the isolated or consecutive application of both stimuli. These findings could be of great interest in order to improve peripheral vascular properties. However, despite the results, it is not possible to clearly establish whether an acute increase in peripheral artery blood flow would prevent and/or heal pressure ulcers in individuals with SCI. Future studies are warranted to examine the chronic efficacy of WBV+ES on peripheral artery function in SCI patients. Information about optimal dose of WBV+ES and time of delivery should be evaluated.
Data archiving
There were no data to deposit.
References
Jacobs PL, Nash MS . Exercise recommendations for individuals with spinal cord injury. Sports Med 2004; 34: 727–751.
Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005; 43: 408–416.
D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–753.
Krum H, Howes LG, Brown DJ, Ungar G, Moore P, McNeil JJ et al. Risk factors for cardiovascular disease in chronic spinal cord injury patients. Paraplegia 1992; 30: 381–388.
West CR, Alyahya A, Laher I, Krassioukov A . Peripheral vascular function in spinal cord injury: a systematic review. Spinal Cord 2013; 51: 10–19.
Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS . Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med 2000; 23: 129–135.
Krause JS, Saunders LL . Health, secondary conditions, and life expectancy after spinal cord injury. Arch Phys Med Rehabil 2011; 92: 1770–1775.
De Groot PC, Van Kuppevelt DH, Pons C, Snoek G, Van Der Woude LH, Hopman MT . Time course of arterial vascular adaptations to inactivity and paralyses in humans. Med Sci Sports Exerc 2003; 35: 1977–1985.
Houtman S, Oeseburg B, Hopman MT . Blood volume and hemoglobin after spinal cord injury. Am J Phys Med Rehabil 2000; 79: 260–265.
Krum H, Howes LG, Brown DJ, Louis WJ . Blood pressure variability in tetraplegic patients with autonomic hyperreflexia. Paraplegia 1989; 27: 284–288.
Phillips AA, Cote AT, Warburton DE . A systematic review of exercise as a therapeutic intervention to improve arterial function in persons living with spinal cord injury. Spinal Cord 2011; 49: 702–714.
Radegran G, Saltin B . Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol 1998; 274: H314–H322.
Prior BM, Yang HT, Terjung RL . What makes vessels grow with exercise training? J Appl Physiol (1985) 2004; 97: 1119–1128.
Prior SJ, McKenzie MJ, Joseph LJ, Ivey FM, Macko RF, Hafer-Macko CE et al. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation 2009; 16: 203–212.
Stebbings GK, Morse CI, McMahon GE, Onambele GL . Resting arterial diameter and blood flow changes with resistance training and detraining in healthy young individuals. J Athl Train 2013; 48: 209–219.
Gobbo M, Maffiuletti NA, Orizio C, Minetto MA . Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J Neuroeng Rehabil 2014; 11: 17.
Vanderthommen M, Depresseux JC, Dauchat L, Degueldre C, Croisier JL, Crielaard JM . Blood flow variation in human muscle during electrically stimulated exercise bouts. Arch Phys Med Rehabil 2002; 83: 936–941.
Babault N, Cometti C, Maffiuletti NA, Deley G . Does electrical stimulation enhance post-exercise performance recovery? Eur J Appl Physiol 2011; 111: 2501–2507.
Olive JL, Slade JM, Dudley GA, McCully KK . Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol (1985) 2003; 94: 701–708.
Lohman EB 3rd, Sackiriyas KS, Bains GS, Calandra G, Lobo C, Nakhro D et al. A comparison of whole body vibration and moist heat on lower extremity skin temperature and skin blood flow in healthy older individuals. Med Sci Monit 2012; 18: CR415–CR424.
Rittweger J, Moss AD, Colier W, Stewart C, Degens H . Muscle tissue oxygenation and VEGF in VO-matched vibration and squatting exercise. Clin Physiol Funct Imaging 2010; 30: 269–278.
Yarar-Fisher C, Pascoe DD, Gladden LB, Quindry JC, Hudson J, Sefton J . Acute physiological effects of whole body vibration (WBV) on central hemodynamics, muscle oxygenation and oxygen consumption in individuals with chronic spinal cord injury. Disabil Rehabil 2014; 36: 136–145.
Herrero AJ, Menendez H, Gil L, Martin J, Martin T, Garcia-Lopez D et al. Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord 2011; 49: 554–559.
Koutnik AP, Wong A, Kalfon R, Madzima TA, Figueroa A . Acute passive vibration reduces arterial stiffness and aortic wave reflection in stroke survivors. Eur J Appl Physiol 2014; 114: 105–111.
Menendez H, Martin-Hernandez J, Ferrero C, Figueroa A, Herrero AJ, Marin PJ . Influence of isolated or simultaneous application of electromyostimulation and vibration on leg blood flow. Eur J Appl Physiol 2015; 115: 1747–1755.
Marin PJ, Bunker D, Rhea MR, Ayllon FN . Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. J Strength Cond Res 2009; 23: 2311–2316.
Fuller JT, Thomson RL, Howe PR, Buckley JD . Effect of vibration on muscle perfusion: a systematic review. Clin Physiol Funct Imaging 2013; 33: 1–10.
Bovenzi M, Welsh AJ, Griffin MJ . Acute effects of continuous and intermittent vibration on finger circulation. Int Arch Occup Environ Health 2004; 77: 255–263.
Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA . The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther 2009; 11: 39–43.
Lythgo N, Eser P, de Groot P, Galea M . Whole-body vibration dosage alters leg blood flow. Clin Physiol Funct Imaging 2009; 29: 53–59.
Maffiuletti NA . Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol 2010; 110: 223–234.
Olive JL, Dudley GA, McCully KK . Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 2003; 35: 901–907.
Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ et al. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 2008; 586: 1137–1145.
Rowley NJ, Dawson EA, Hopman MT, George KP, Whyte GP, Thijssen DH et al. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc 2012; 44: 844–849.
Lohman EB 3rd, Bains GS, Lohman T, DeLeon M, Petrofsky JS . A comparison of the effect of a variety of thermal and vibratory modalities on skin temperature and blood flow in healthy volunteers. Med Sci Monit 2011; 17: MT72–MT81.
Cochrane DJ, Stannard SR, Walmsely A, Firth EC . The acute effect of vibration exercise on concentric muscular characteristics. J Sci Med Sport 2008; 11: 527–534.
Lohman EB 3rd, Petrofsky JS, Maloney-Hinds C, Betts-Schwab H, Thorpe D . The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med Sci Monit 2007; 13: CR71–CR76.
Acknowledgements
We thank the Ministry of Education of the Government of Castilla y León for the funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Menéndez, H., Ferrero, C., Martín-Hernández, J. et al. Acute effects of simultaneous electromyostimulation and vibration on leg blood flow in spinal cord injury. Spinal Cord 54, 383–389 (2016). https://doi.org/10.1038/sc.2015.181
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.181
This article is cited by
-
Non-pharmacological interventions for vascular health and the role of the endothelium
European Journal of Applied Physiology (2022)
-
A Review of Functional Electrical Stimulation Treatment in Spinal Cord Injury
NeuroMolecular Medicine (2020)
-
Whole Body Vibration for People with Spinal Cord Injury: a review
Current Physical Medicine and Rehabilitation Reports (2017)
-
Chronic effects of simultaneous electromyostimulation and vibration on leg blood flow in spinal cord injury
Spinal Cord (2016)