Abstract
Study design:
Retrospective case-control study
Objectives:
The intent of this study was to investigate the relationships between vertebral degenerative changes resulting in spinal canal stenosis, spinal cord lesions and the development of spinal cord decompression sickness (DCS) in scuba divers.
Setting:
Referral hyperbaric facility, Toulon, France.
Methods:
We examined 33 injured divers less than 50 years old by cervical and thoracic MRI and compared them with 34 matched control divers. The number of intervertebral disk abnormalities and the degree of canal compression were analyzed on T2-weighted sagittal images using a validated grading system developed recently. The presence and the distribution of hyperintense cord lesions in relation with the accident and the recovery status at 6 months were also assessed.
Results:
Canal spinal narrowing was more common in injured divers than in controls (79% vs 50%, OR=3.7 [95% CI, 1.3–10.8], P=0.021). We found a significant linear association between the extent of canal stenosis, multisegmental findings and the development of spinal cord decompression sickness. MRI intramedullary lesions were significantly more frequent in divers with incomplete recovery (OR=16 [95% CI, 2.6–99], P=0.0014), but statistical analysis failed to demonstrate a significant relationship between canal compression, signal cord abnormalities and a negative clinical outcome.
Conclusions:
These results suggest that divers with cervical and thoracic spinal canal stenosis, mainly due to disk degeneration, are at increased risk for the occurrence of spinal cord decompression sickness.
Similar content being viewed by others
Introduction
Decompression sickness (DCS) is an acute but rare disorder affecting scuba divers. This pathological condition results originally from the excessive formation of gas bubbles in blood and tissues during ascent and after surfacing. Injuries to the central nervous system are predominant in severe DCS, and the spinal cord is the most commonly affected site. The clinical features of spinal involvement are numerous, and presenting symptoms may vary considerably from minimal subjective sensory abnormalities to more severe neurological deficits leading to incomplete recovery with potential permanent disability in 20–30% of patients.1, 2 The pathophysiological mechanisms of spinal cord lesions are not yet completely understood and include several hypotheses, that is, venous infarction, autochthonous bubble formation and arterial gas embolism. Although the prevalent theory of this myelopathy is based on a pure venous pattern resulting from bubble embolization of the epidural vertebral venous system with venous drainage obstruction and subsequent coagulation activation,3 recent MRI studies suggest that spinal cord DCS may be a combination of both arterial and venous infarction.4, 5
Currently, individual risk factors for DCS development are not strictly determined in part to the great inter/intravariability between divers regarding susceptibility to DCS. Besides the most predisposing factor particularly studied in the past 2 decades that is patent foramen ovale,6, 7 other parameters such as ageing, increased amount of body fat and low aerobic fitness were found to increase the risk of DCS.8, 9
Narrowing of the spinal canal may be caused by various factors, including disk herniation, osteophyte formation and ossification of the vertebral ligaments; MRI is by far the most commonly used imaging method for the accurate evaluation of the spinal cord and adjacent vertebral structures. These degenerative findings increasing linearly with age are frequently recognized in asymptomatic subjects.10, 11 However, it has been recently documented with MRI that these morphologic changes may lead to progressive spinal cord compression, resulting in the development of ischemic myelopathy, particularly in the cervical area of elderly patients.12, 13 To our knowledge, only one study examined the role of intervertebral disk abnormalities and other spinal canal compressive factors as predictors of severe spinal cord DCS in divers exhibiting T2-weighted hyperintense cord signal changes on MRI.14 We postulate that cervical and thoracic spinal canal stenosis may be a risk factor for divers experiencing neurological DCS by decreasing spinal blood flow and/or by altering spinal cord off-gassing and epidural venous drainage, leading to perifocal ischemia and lastly to spinal cord infarction. Therefore, we carried out a controlled MRI study to investigate the association between the degree of canal compression and the development of spinal cord DCS. We also sought to assess the relationships between radiological findings and the clinical outcome.
Materials and methods
Patients
Between 2009 and 2012, 33 recreational scuba divers treated in our hyperbaric facility for spinal cord DCS were retrospectively enrolled in the present study. Patients were consecutively eligible for inclusion only if they were less than 50 years old and underwent an MRI examination of the cervical and thoracic spine within 1 month of injury. Clinical diagnosis of spinal cord DCS was made when the criteria of bilateral sensory or/and motor deficit were recognized within six hours of surfacing of a dive. Cases with unilateral neurological symptoms but presenting other characteristic signs consistent with spinal cord involvement in DCS such as acute back pain or bladder dysfunction were also included. Patients with ambiguous presentation (headache, transient tingling and minor nondermatomal paresthesias, fatigue, feeling of malaise) and believed to have cerebral or inner ear DCS (vertigo, nausea, visual disturbance, altered higher function or speech) were excluded. Clinical outcome was determined by the recovery status at 6 months postinjury and dichotomized into 2 categories: divers with full recovery and those with residual neurological symptoms defined as persistent objective sensory, motor or urinary/bowel disorders.
The control group consisted of 34 healthy recreational divers recruited from local diving clubs matched with respect to age, gender, diving experience, body mass index, history of spinal trauma and surgery, and smoking habits (Table 1).
We certify that all applicable institutional regulations concerning the ethical use of human volunteers were followed during the course of this research (approval by the local ethics committee and signed informed consent prior to entering the study).
MRI examination
The MRI scans were performed with a 3-T scanner (HDXT, General Electric Healthcare, Milwaukee, Wisconsin, USA) using a 8-channel spine (CTL) coil, with the patient in the supine position. The imaging protocol included sagittal T2-weighted Fast Spin Echo images, acquired with the following parameters: TR/TE, 3000–3120/100–120; field of view, 320–320 mm; section thickness 3 mm with a 0.3 mm gap; matrix 320*320 mm. When hyperintense lesions were suspected in sagittal planes, the axial T2-weighted images were used as a supplementary evaluation method. Eight of out 33 DCS divers were examined with 1.5-T MRI using different protocols from outside hospitals. The imported scans were recorded on a computer and T2-weighted sagittal images were then selected for grading.
The cervical and thoracic intervertebral levels from C2-C3 to T11-T12 were quantitatively and qualitatively analyzed. The presence and degree of spinal canal stenosis at the maximal narrowing point was determined in accordance with a new grading system proposed by Kang et al.15 and validated afterwards in another study assessing the correlation between MRI grade and the clinical manifestations of cervical spinal compression.13 Canal stenosis was classified using the following grades on the basis of T2-weighted midsagittal images: grade 0 indicated the subarachnoid space≤50%; grade 1, subarachnoid space>50% without cord deformity; grade 2, spinal cord deformity without cord signal change; and grade 3, severe spinal cord compression with focal cord signal change near the compressed level corresponding to a sign of underlying spondylotic myelopathy. In addition, all other pathological MRI findings, such as hyperintense lesions indicative of spinal cord infarction and abnormal vertebral bodies, were recorded. The sacral cord and conus medullaris were not explored since DCS injuries below L1 vertebrae have never been radiologically reported.
The MRI films were read by 1 experienced neuroradiologists (T.L.) who was blinded to the pathological condition or not of the divers. The images were interpreted twice with an interval of 15 days, to minimize observer variability.
Statistical analysis
Continuous data are expressed as mean±s.d. after passing the normality test. Categorical variables between groups were compared using the χ2 test for linear trend or the Fisher’s exact test where appropriate, and comparisons between continuous variables were done with the unpaired t test. Odds ratios with 95% confident intervals were calculated when needed, with the P values, for which the level of significance was set as<0.05. The statistical analysis was performed with Graphpad Prism, version 5.00 (Graphpad Software, San Diego, California, USA).
Results
A total of 69 intervertebral disk abnormalities⩾grade 1 were seen in 26 injured divers (79%) while 34 intervertebral disk abnormalities⩾grade 1 were detected in 17 controls (50%). Canal stenosis were mostly on the cervical level (C5-C6, C6-C7 and C4-C5 in descending order of importance) with 54 disks degeneration in the DCS group compared with 29 in the control group. Nine injured divers had a narrowing of the spinal canal affecting both the cervical and thoracic parts of the spine versus 4 controls.
Only 1 control diver exhibited a clinically asymptomatic grade 3 cervical canal stenosis and was grouped with grade 2 for the purposes of this study. Otherwise, 2 DCS divers presented radiological sequelae of vertebral body thoracic trauma with adjacent disk protrusion classified as grade 1 and 2, respectively.
The proportion of DCS divers as a function of the grading system as well as the total number of intervertebral disks abnormalities found in each patient are presented in Tables 2 and 3. Statistical analysis revealed a strong and linear association between the degree of spinal canal compression, multisegmental findings and the occurrence of spinal cord DCS (P=0.0031 and P=0.0033, respectively). When comparing the subset of patients without canal stenosis (grade 0) and those with at least one disk degeneration⩾grade 1, we found that obliteration of the subarachnoid space was significantly more frequent in our population of DCS divers than in controls (79% vs 50%, OR=3.7 [95% CI, 1.3–10.8], P=0.021). The analysis of the distributions of canal stenosis and the highest dermatomal level of original neurological impairment (determined by the level of sensory disturbance or acute back pain) revealed that 17 out of the 26 patients with spinal cord narrowing presented a strong relationship between the clinical level and one of the sites of compression (8 cases at the cervical region and 9 at the thoracic region, respectively). The remaining 9 patients all had cervical spinal canal stenosis of varying importance with neurological symptoms localized below the anatomical level of compression at a distance ranged from 5–13 vertebral segments (Figure 1).
There were 15 out of 33 injured divers who had incomplete recovery after 6 months. Among them, 10 displayed extensive or focal patchy areas of T2 hyperintensities in the spinal cord after early MRI examination (i.e., during the first week following diving injury). Conversely, only 2 patients out of 18 with full recovery had signal abnormalities, thus giving a higher prevalence of demonstrable cord damages in divers with the poorer outcome (OR=16 [95% CI, 2.6–99], P=0.0014). It is interesting to note that these 2 divers have presented severe symptoms at admission and have maintained mild residual deficits during 1 month before complete resolution. The distribution of lesions was mainly in the white matter of the posterior and lateral part of the column, variably affecting the cervical and/or thoracic cord segments. 2 patients demonstrated involvement of both gray and white matter and another 1 patient exhibited MRI lesions at 2 different levels of the spinal cord. Among the 12 divers with positive MRI, 9 showed agreement between the initial neurologic symptoms and the localization of hyperintense images, and 10 of them had at least 1 abnormal disk graded 1 or 2 in connection with one of the sites of spinal cord lesions (Figures 2 and 3). Statistics, however, failed to demonstrate a significant relationship between the presence of canal stenosis, signal cord abnormalities and an unfavourable clinical outcome.
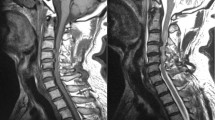
41-year-old male diver with spinal cord decompression sickness. Sagittal T2-weighted MR image showing hyperintense signal involving the thoracic spine from caudal T3 to T5 level. Spinal canal is narrow at T5-T6 level and T8-T9 (grade 2 stenosis). Note the Schmorl’s nodes in the body of T7 and T8 with surrounding high signal intensity indicating the presence of oedema in the vertebral bone marrow. Close questioning of the patient revealed a previous history of thoracic trauma several years ago.
Discussion
For the first time, our results tend to indicate that reduction in sagittal spinal canal diameter mainly due to disk degeneration and osteophytic spurs increase the likelihood of spinal cord DCS in scuba divers. These findings are particularly obvious at the cervical level and are closely related to the extent of the compression and the multisegmental involvement.
It is generally assumed that spinal cord damages due to DCS are mainly of vascular origin. Experimental and autopsy studies demonstrating abnormal histological findings, such as punctuate hemorrhages, gray matter necrosis, axonal swelling and venous congestion with activation of coagulation and presence of intravascular bubbles, have led to the conclusion that global ischemia due to occlusion of the arterial or venous system or both may account for this disease. In addition, formation of autochthonous bubbles within the lipid-rich myelin may also contribute to these phenomena by inducing tissue laceration and stretching.16
In the present study, the relationship between the predominant compression in the anterior cord and the finding that most lesions are in the posterior portion of the cord strengthens the notion that the pathogenesis of spinal cord DCS is rather attributed to a vascular mechanism than a direct injury on the neural tissue. We propose that static mechanical factors combined with dynamic forces may compromise spinal cord vascularisation and may increase strain and shear forces applied on the spine, causing arterial hypoperfusion and impairment of venous outflow in the epidural venous system. These ischemic conditions may promote the accumulation of nitrogen bubbles in situ, but also initiate a complex cascade of biomolecular changes, including release of inflammatory mediators, excitotoxicity and apoptosis, finally leading to demyelinisation, gliosis and necrosis, as already described in the myelopathy of cervical spondylosis.17
Our data describe a good agreement between the initial neurological symptoms and the level of at least one of the site of spinal canal narrowing for the majority of injured divers; but, in some cases, there was a mismatch between the localization of intervertebral disk abnormalities and the clinical dermatomal level distal to the spinal cord compression. This phenomenon is not unusual in spinal cord pathology with demyelinating lesions where long tract fibers are frequently involved.18, 19
To date, MRI yields low sensitivity in spinal cord DCS with an incidence of detected intramedullary lesions ranging from 12–31% in the existing literature comprising small series.14, 20, 21, 22 This is essentially due to technical insufficiency to detect small detailed patchy pathologic changes. Nevertheless, the current results are in line with our own previous work showing that early MRI examination of the spinal cord may provide useful prognostic information in the prediction of mid-term neurological sequelae following spinal cord DCS. Indeed, in a prior series of 45 spinal cord DCS divers treated between 2002 and 2007, we have shown that there was a strong correlation between MRI lesions, the initial severity on admission and the presence of residual deficits at 1 month.14 However, the lack of signal abnormalities does not necessarily mean that the patient will not have a worse outcome since 5 out of 15 DCS divers with incomplete recovery in the present investigation did not exhibit MRI lesions.
Surprisingly, we did not confirm the additional findings observed in our first report,14 demonstrating that signal cord changes were significantly related to spinal cord impingement, although 83% of divers with MRI lesions here presented vertebral abnormalities on contact with the spinal cord near the site of the myelopathy. We cannot exclude that this discrepancy may be due to the lack of statistical power because of the small sample size of injured divers and also the high prevalence of intervertebral disks degeneration detected in the present study. It is noteworthy that the proportion of controls with canal stenosis was not different from another cohort of 40 years-old healthy divers examined with MRI of the cervical and thoracic spine (50 vs 58%, respectively), excluding a selection bias with potentially fewer disk abnormalities in our participants.23 We must admit, however, that the prevalence of compressive factors in this series of spinal cord DCS divers (79%) was much higher than that we described previously (33%).14 We may presume that this discordant statement between the 2 investigations is because the present results were extracted from a standardized grading system, while prior results were assessed with a more simplistic classification, thus limiting the corresponding reproducibility between findings. A potential sampling bias with overrepresentation of injured divers presenting degenerative disk changes in this report is another possibility.
Conclusion
The new finding of this study is that intervertebral disk changes and other vertebral abnormalities resulting in spinal canal stenosis are potentially predisposing factors for the development of spinal cord DCS in divers. The link between these compressive disorders and the severity of the dysbaric myelopathy remains to be established. Since our findings may have future clinical implications for recommending against diving in patients who are known to have a history of degenerative spinal canal disorders, further studies are needed to validate these preliminary results.
Data archiving
There were no data to deposit.
References
Gempp E, Blatteau JE . Risk factors and treatment outcome in scuba divers with spinal cord decompression sickness. J Crit Care 2010; 25: 236–242.
Aharon-Peretz J, Adir Y, Gordon CR, Kol S, Gal N, Melamed Y . Spinal cord decompression sickness in sport diving. Arch Neurol 1993; 50: 753–756.
Hallenbeck JM, Bove AA, Elliott DH . Mechanisms underlying spinal cord damage in decompression sickness. Neurology 1975; 25: 308–316.
Louge P, Gempp E, Hugon M . MRI features of spinal cord decompression sickness presenting as Brown-Sequard syndrome. Diving Hyperb Med 2012; 42: 88–91.
Hennedige T, Chow W, Ng YY, Chung-Tsing GC, Lim TC, Kei PL . MRI in spinal cord decompression sickness. J Med Imaging Rad Onc 2012; 56: 282–288.
Lairez O, Cournot M, Minville V, Roncalli J, Austruy J, Elbaz M et al. Risk of neurological decompression sickness in the diver with right-to left shunt: Literature review and meta-analysis. Clin J Sport Med 2009; 19: 231–235.
Gempp E, Blatteau JE, Stephant E, Louge P . Relation between right-to-left shunts and spinal cord decompression sickness in divers. Int J Sports Med 2009; 30: 150–153.
Carturan D, Boussuges A, Vanuxem P, Bar-Hen A, Burnet H, Gardette B . Ascent rate, maximal oxygen uptake, adiposity, and circulating venous bubbles after diving. J Appl Physiol 2002; 93: 1349–1356.
Smertz RW . Age associated risks of recreational scuba diving. Diving Hyperb Med 2007; 37: 162–163.
Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y et al. Age-related changes of thoracic and cervical intervertebral discs in asymptomatic subjects. Spine 2010; 35: 1359–1364.
Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S . Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990; 72: 1178–1184.
Harrop JS, Naroji S, Maltenfort M, Anderson DG, Albert T, Ratliff JK et al. Cervical myelopathy: a clinical and radiographic evaluation and correlation to cervical spondylitic myelopathy. Spine 2010; 35: 620–624.
Park HJ, Kim SS, Chung EC, Lee SY, Park NH, Rho MH et al. Clinical correlation of a new practical MRI method for assessing cervical spinal canal compression. Am J Roentgenol 2012; 99: W197–W201.
Gempp E, Blatteau JE, Stephant E, Pontier JM, Constantin P, Pény C . MRI findings and clinical outcome in 45 divers with spinal cord decompression sickness. Aviat Space Environ Med 2008; 79: 1112–1116.
Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW et al. New MRI grading system for the cervical canal stenosis. Am J Roentgenol 2011; 197: W134–W140.
Francis TJR, Mitchell SJ . Pathophysiology of decompression sickness. In: Brubbak AO, Neuman TS (eds). The Bennett and Elliot’s Physiology and Medicine of Diving 5th edn. WB Saunders: London. 2003 pp 530–556.
Baptiste DC, Fehlings MG . Pathophysiology of cervical myelopathy. Spine J 2006; 6: 190S–197S.
Adams KK, Jackson CE, Rauch RA, Hart SF, Kleinguenther RS, Barohn RJ . Cervical myelopathy with false localizing sensory levels. Arch Neurol 1996; 53: 1155–1158.
Hellmann MA, Djaldetti R, Luckman J, Dabby R . Thoracic sensory level as a false localizing sign in cervical spinal cord and brain lesions. Clin Neurol Neurosurg 2013; 115: 54–56.
Warren LP, Djang WT, Moon RE, Camporesi EM, Skip SD, Anthony DC et al. Neuroimaging of scuba diving injuries to the CNS. Am J Roentgenol 1988; 151: 1003–1008.
Reuter M, Tetslaff K, Hutzelmann A, Fritsch G, Steffens JC, Bettinghausen E et al. MRI of the CNS in diving-related decompression illness. Acta Radiol 1997; 38: 940–944.
Tournebise H, Boucand MH, Landi J, Theobald X . Paraplegia and decompression sickness. Paraplegia 1995; 33: 636–639.
Bartsch T, Cordes P, Keil R, Reuter M, Hutzelmann A, Tetzlaff K et al. Cervico-thoracic disc protrusions in controlled compressed-air diving: clinical and MRI findings. J Neurol 2001; 6: 514–516.
Acknowledgements
The authors thank the volunteer divers for their participation in this study. This work would not have been possible without the assistance of the imaging technicians and the support of C Arteaga, Professor, Head of Radiology and Diagnostic Imaging Department at the Sainte Anne’s military hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gempp, E., Louge, P., Lafolie, T. et al. Relation between cervical and thoracic spinal canal stenosis and the development of spinal cord decompression sickness in recreational scuba divers. Spinal Cord 52, 236–240 (2014). https://doi.org/10.1038/sc.2013.121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.121
Keywords
This article is cited by
-
Thorakaler Diskusprolaps – oft falsch eingeschätzt?
NeuroTransmitter (2016)