Abstract
Study design:
Pilot study.
Objectives:
The aim of the study was to develop a neurophysiological method to diagnose the cranial as well as the caudal level of a complete thoracic spinal cord injury (SCI) with higher precision than today's protocols.
Setting:
SCI unit Karolinska University Hospital, Stockholm, Sweden.
Methods:
Bipolar needle electromyography was recorded in intercostal spaces of five patients with chronic, complete thoracic SCI. Tests were performed during rest, during voluntary activation and during activation of lower body spasticity. Magnetic resonance imaging (MRI) was performed in each patient according to a protocol optimized for imaging near metal implants.
Results:
Three distinct patterns were found in each patient. Above the lesion we found voluntary activated, normal motor unit potentials (MUPs). At the neurological level and a varying number of segments below, denervated intercostal segments with fibrillation potentials and positive sharp waves appeared. Below the neurological level, normal MUP activated in concert with lower body spasticity was found. The number of denervated segments showed a significant correlation to the length of spinal cord discontinuity on MRI (r=0.97, P<0.05).
Conclusion:
Intercostal neurophysiology in combination with MRI optimized for imaging near metal implants can be used to determine the extent of a chronic complete thoracic SCI, both anatomically and functionally. The described method increases the sensitivity to detect delicate neurological changes related to the dynamic of the pathology that follows SCI and may be useful in analyzing outcome in clinical trials.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) with loss of neurological function represents a major challenge, not only in terms of adjusting patients and their environment to daily life with SCI, but also on a basic scientific level in developing therapeutic options that improve outcome in patients. A number of strategies are being investigated, and some are approaching the clinic (Kwon et al.1 for an overview). In any clinical trial treatment safety is the most important concern. For this reason, the International Campaign for Cures of spinal cord injury Paralysis (ICCP) guidelines for clinical trials refer to patients with complete (American Spinal Injury Association (ASIA) Impairment Scale (AIS) A) lesions in the thoracic spinal cord as the preferred patient group for clinical trials.2, 3, 4, 5 Local adverse effects of a therapeutic strategy in these patients would be less likely to deteriorate function. On the other hand, the ICCP panel conclude that no widely accepted method for assessing local function of the thoracic spinal cord exists other than sensory evaluation according to the ASIA protocol. Change in sensory level over time in complete thoracic SCI has been investigated to determine what should be considered a significant deterioration or improvement.6, 7 These studies suggest that a change in sensory level of three dermatomes should be considered a rare event and could be used to track safety.
A comprehensive review of clinical modalities for assessment of sensory, motor and autonomic function in thoracic SCI is presented in Ellaway et al.8 Further advances regarding motor function was reviewed in 2007.9 Motor evoked potentials of the paraspinal muscles10 as well as intercostal muscles11 is described as a means of evaluating motor function in thoracic SCI. Kuppuswamy et al.10 note that erector spinae muscles extend more than one vertebral segment making them less selective for evaluation of the thoracic cord. Electromyography (EMG) signal could be elicited several segments below the injury level, this was not seen in intercostal muscles.11 Anatomical studies of human intercostal muscles and innervation also support their isolated segmentation.12 Every intercostal space was shown to be innervated by its own intercostal nerve, the medial branch of the spinal nerve and the muscle itself isolated between ribs.12
The aim of this study was to develop a neurophysiological method to evaluate motor function in the thoracic spinal cord with better precision than today's protocols. We hypothesize that intercostal EMG activity reflects the state of the corresponding motor neuron pool in the spinal cord. Further, the magnetic resonance imaging (MRI) sequence used in this protocol was optimized to obtain imaging of the spinal cord near titanium implants.
Materials and methods
Patients
Approval for the study protocol was acquired from the local human ethics committee at the Karolinska Institute. Inclusion criteria were male patients 18–65 years, SCI in the thoracic cord from non-penetrating trauma, ASIA A, >1 year after injury. Exclusion criteria included contraindications for MRI such as pacemaker, free metal fragments or self-reported claustrophobia.
Included patients’ electronic medical records were checked for ASIA class from injury to chronic stage (1–4 years) to ensure that no transitions concerning their ASIA classification had taken place.
Physical examination
A neurological examination was performed according to the ASIA protocol in each patient by the same senior neurologist prior to each neurophysiological exam, to determine the clinical injury level and the presence of spasticity.
Neurophysiology
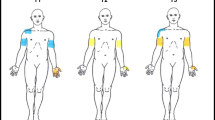
All examinations were performed by the same senior neurophysiologist, blinded to the results from the clinical examination and MRI. Two clinicians counted intercostal spaces, and validation was performed through placement of an indicator between two ribs during MRI examination. EMG recordings were obtained bilaterally with a bipolar needle from the intercostal muscles in rest, during voluntary activation through head lift (assessment of the cranial border of the spinal cord lesion) and spastic activation of lower limbs (assessment of the caudal border of the spinal cord lesion). Care was taken to exclude the pectoralis major and serratus anterior muscles. Needle placement was selected (Figure 1), and specific activation of pectoralis major and serratus anterior muscles was performed to evaluate their contribution in every specific needle position.
Neurophysiological results were translated into number of denervated segments. This was defined as the number of whole spinal segments showing complete denervation. When denervation was unilateral in one segment, or if one segment presented a mixed result, it was considered as 0.5 denervated segments. The patients’ sensation of the needle insertion was also recorded.
MRI
All patients underwent a 1.5T MRI exam using a whole-body MRI scanner (Philips Intera Master, Best, The Netherlands) with a 15 ch spine coil (Medical Advances, Milwaukee, WI, USA). All MRI examinations were performed according to the same protocol. A three-plane localizer was obtained covering the spinal canal. The diagnostic imaging protocol was acquired in a sagittal plane using a customized 3D TSE T2W isotropic voxel pulse sequence. The voxel size was 1.0 × 1.0 × 1.0 mm with the following parameters: repetition time 2000 ms, echo time 120 ms, flip angle 90 ms, echo train length 97 ms, profile order linear Y, field of view 300 mm, bandwidth 653 Hz, flow compensation sensitized, fold-over direction anteroposterior (AP) and number of signal averaged 1. Multiplanar reconstruction was performed on a Philips workstation using Philips Easy vision R3.5.
To be able to securely determine the dimension of the spinal cord in the MRIs, a saline immersed paraffin phantom cast as a piston with a known outer diameter was added to every examination.
The length of the discontinuity of the spinal cord was determined by cranio-caudal examination of the spinal cord in the transverse plane. The cranial marker was set where signs of neural tissue and exiting spinal nerves disappeared and the caudal marker where these signs reappeared.
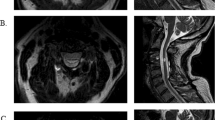
The sagittal multiplanar reconstruction images shown in Figure 4 were scaled to the same relative size using Adobe Photoshop CS4. Rotation, brightness and contrast were also adjusted to facilitate comparison.
(a–e) Sagittal TSE T2-weighted MR images of 5 sensorimotor complete thoracic SCI patients. Multiplanar reconstruction was performed with the anterior aspect of the spinal cord as midline, allowing for one sagittal image showing the whole spinal cord despite scoliosis in the patient. All patients had their spines stabilized with titanium instrumentation. Same letters assigned as in Figure 3. Patient (a) showed a complete discontinuity of the spinal cord over vertebrae T6–T7, estimated to 55 mm. Patient (b) had a more complex injury, with adhesions of the spinal cord to the dural sac at the proximal side of the injury, and a syringomyelia at the distal end. The discontinuity over T6–T7 was measured to 60 mm. Patient (c) presented with a complete lesion with a central cyst at T5 measuring 23 mm. Patient (d) had a marked atrophy of the cranial spinal cord and several segments caudal of the injury affected by a syringomyelia. The discontinuity of the cord over T3–T4 was estimated to 30 mm. Patient (e) had a complete lesion at T5 with a central cyst measuring 14 mm.
Statistics
The Pearson product-moment correlation coefficient was calculated in Microsoft Excel.
Statements of Ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Results
Five male patients with complete chronic SCI in the thoracic cord were included. Written and informed consent was acquired from all subjects. Age of patients was 32–50 years (median 35), 1–4 years post-injury (median 3). Three patients were injured in motorcycle accidents, one patient in a motor vehicle accident and one in an accident with a hang-glider. All patients presented as AISA A and remained so throughout the clinical course. None of the patients had a clinical history of frequent autonomic dysreflexia.
Clinical examination
Patients underwent clinical examination to confirm that all inclusion criteria were met. They were all determined ASIA A as their clinical history suggested. Neurological level was T3–T6 (median T5), and lower body spasticity was present in all patients.
Neurophysiology
To assess motor function of the thoracic spinal cord, bipolar needle EMG registrations in the intercostal muscles were performed. Three distinct patterns were recognized: Above the level of SCI normal, voluntarily activated motor unit potentials appeared (Figure 2a). At, or close to, the level of sensory loss patients presented a varying number of intercostal spaces with spontaneous EMG activity with fibrillation potentials and positive sharp waves (Figure 2b). Below the level of injury there were once again normal motor unit potentials (Figure 2c), generated in concert with spastic activation of lower limbs. The registrations and number of denervated segments from the five individual patients are presented in Figure 3.
Representative recordings of intercostal electromyography (EMG) in sensorimotor complete SCI patients showing three distinct patterns. (a) Voluntarily activated, typical motor unit potential obtained in intercostal muscle above the level of injury. (b) EMG recording in intercostal muscle from the lesion area showing signs of denervation with positive sharp waves and fibrillation potentials. No motor unit potentials (MUPs). (c) Registration from paretic muscles without voluntary activation showed normal MUP after spastic activation of the lower part of the body. (d) Myotomes with large amplitude reflected reinnervation and was interpreted as previous root injury.
Results from intercostal electromyography examination of five sensorimotor complete thoracic SCI patients. Sensory levels are indicated. Some segments showed a mixed pattern (*). Same letters assigned as in Figure 4. Patient (a) showed two full segments of denervation activity before spastically activated normal motor unit potential (MUP) reappeared below the lesion. Patient (b) had voluntarily activated large-amplitude MUP just adjacent to the lesion interpreted as a reinnervated root injury, and an asymmetrical reappearance of spastically activated normal MUP, totaling 2.25 denervated segments. Patient (c) showed no denervated segment. Patient (d) had an asymmetrical injury translated to 0.75 denervated segments, in one segment below the lesion spastically activated large amplitude MUP were found interpreted as an earlier root injury. Patient (e) presented with one unilateral segment showing a mixed pattern of voluntarily activated MUP and denervation activity, 0.25 denervated segments in total.
The patients’ sensation of the needle insertion was used to determine the sensory level. It was coherent with motor level in two patients, one segment was found above the voluntary motor level in two patients, and one segment below the motor level in one patient (Figure 3).
MRI
MRI was performed in all patients to give an anatomical overview of the individual injury and facilitate interpretation of results from neurophysiology. In this sample of five ASIA A thoracic SCI patients, the anatomical extent of the SCI varied. The length of spinal cord discontinuity was 13–60 mm, with the median found at 30 mm (Figure 4).
All patients had their spine previously internally stabilized with titanium instrumentation.
Comparing neurophysiology and MRI
The number of denervated segments was plotted against the length of the spinal cord discontinuity as judged by MRI (Figure 5). The Pearson product-moment correlation coefficient for number of denervated segments and length of lesion was r=0.97, P<0.01.
Discussion
The present report suggests that a traditional but thoroughly performed neurophysiological examination may provide a detailed map of the functional cranial and caudal margins of a thoracic SCI in patients. By activating the motor neurons in the ventral horn, EMG registrations from corresponding myotomes reflect the viability of the spinal cord. Cranial to the injury, motor neurons were activated voluntarily, whereas caudal to the injury, motor neurons were activated by inducing spasticity in the lower part of the body, a strategy that to our knowledge has not previously been described. Further, we present an MRI sequence for spinal cord imaging that delineates the injury zone with a high correlation (r=0.97) to the functional evaluation.
The injury zone showed a denervation pattern with positive sharp waves and fibrillation potentials. This pattern is thought to arise from spontaneous firing from upregulated acetyl choline receptors a few weeks after stripped neural input, which remains until reinnervation occurs.13 The patients in this study presented with a permanent denervation pattern in the injury zone, without signs of reinnervation. This finding was interpreted as devitalized motor neurons in the corresponding ventral horn and thus chronically injured spinal cord tissue, although components of simultaneous peripheral nerve injury cannot be ruled out.
Thoracic motor neuron pools in incomplete SCI (non-ASIA A) could be evaluated just as well as complete (ASIA A). The method will not discriminate between different ASIA classes, as it does not target long fiber tracts crossing the injury zone.
A limitation to our approach is that it is unable to assess sensory and autonomic functions. For a complete evaluation of an SCI, the neurophysiological principles described in this paper needs to be combined with other methods (for discussion see Ellaway et al.8).
No adverse events were seen in our study of five patients. Needle EMG of the intercostal muscles could possibly result in pneumothorax if the needle is inserted too deeply, and has been reported in a few cases of cervical root stimulation.14 We advocate that intercostal needle EMG should be performed by experienced neurophysiologists. According to local preferences, ultrasound could be used for visualization of needle position.
In summary, we report a neurophysiological approach to establish the cranial and caudal margins of motor neuron function in complete thoracic SCI. We suggest that this method would provide a valuable outcome measure in clinical trials. The method may serve as a complement to routine clinical evaluation and MRI in patients with thoracic SCI.
Data Archiving
There were no data to deposit.
References
Kwon BK, Sekhon LH, Fehlings MG . Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine 2010; 35: S263–S270.
Lammertse D, Tuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 2007; 45: 232–242.
Tuszynski MH, Steeves JD, Fawcett JW, Lammertse D, Kalichman M, Rask C et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord 2007; 45: 222–231.
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007; 45: 190–205.
Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221.
Zariffa J, Kramer JL, Fawcett JW, Lammertse DP, Blight AR, Guest J et al. Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord 2011; 49: 463–471.
Harrop JS, Maltenfort MG, Geisler FH, Coleman W, Jones LA, Wirth E et al. Traumatic thoracic ASIA A examinations and potential for clinical trials. Spine 2009; 34: 2525–2529.
Ellaway PH, Anand P, Bergstrom EM, Catley M, Davey NJ, Frankel HL et al. Towards improved clinical and physiological assessments of recovery in spinal cord injury: a clinical initiative. Spinal Cord 2004; 42: 325–337.
Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL et al. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev 2007; 44: 69–76.
Kuppuswamy A, Theodorou S, Catley M, Strutton PH, Ellaway PH, McGregor AH et al. Motor neurone excitability in back muscles assessed using mechanically evoked reflexes in spinal cord injured patients. J Neurol Neurosurg Psychiatry 2005; 76: 1259–1263.
Theodorou S, Catley M, Strutton PH, Davey NJ . Examination of intercostal muscle facilitation evoked by transcranial magnetic stimulation (TMS) in man [abstract]. J Physiol. 2003; 547P: C144.
Sakamoto H, Akita K, Sato T . An anatomical analysis of the relationships between the intercostal nerves and the thoracic and abdominal muscles in man. I. Ramification of the intercostal nerves. Acta Anat (Basel) 1996; 156: 132–142.
Brown WF, Bolton CF, Aminoff MJ . Neuromuscular Function and Disease: Basic, Clinical, and Electrodiagnostic Aspects, Vol 1. Saunders: Philadelphia, 2002.
Hawley RJ . Preventing complications of electromyography. Electromyogr Clin Neurophysiol 2000; 40: 323–325.
Acknowledgements
The work was partly financed through a grant from The Swedish Association of Persons with Neurological Disabilities (Neurologiskt Handikappades Riksförbund).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Frostell, A., Mattsson, P., Persson, J. et al. Neurophysiological evaluation of segmental motor neuron function of the thoracic cord in chronic SCI. Spinal Cord 50, 315–319 (2012). https://doi.org/10.1038/sc.2011.155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.155