Abstract
Background:
Data showing a role for the mid-thoracic spinal cord (SC) in the control of hemodynamic changes is scarce despite existing evidence for its involvement in autonomic regulation.
Study design:
On the basis of the open label prospective series comparing three groups.
Objective:
To determine whether the mid-thoracic SC has a role in hemodynamic regulation during head-up tilt (HUT).
Setting:
Spinal Research Laboratory, Loewenstein Rehabilitation Hospital.
Methods:
A total of 13 healthy control subjects, 10 patients with T4–T6 paraplegia and 11 with C4–C7 tetraplegia were examined during supine rest and during HUT. Heart rate (HR), blood pressure (BP), HR spectral components (lower frequency fluctuation (LF), higher frequency fluctuations (HF) and LF/HF) and cerebral blood flow velocity (CBFV) were continuously measured or calculated.
Results:
BP response to HUT differed among these groups (P<0.02). During HUT, BP decreased markedly in the tetraplegia group (from a mean value of 81.65 to 67.69 mm Hg), and increased in the control groups (from 92.89 to 95.44 mm Hg) and in the T4–T6 paraplegia group (from 96.24 to 97.86 mm Hg). Significant correlation was found in the control and tetraplegia groups between increases in HR LF/HF and HR at HUT (r>0.7; P<0.01). No such correlation was found in the paraplegia group. HUT effect on HR and CBFV was significant in all groups (P<0.001), but group differences were statistically non-significant.
Conclusion:
Findings were generally compatible with those of comparable previously published studies, but they also support a role for the mid-thoracic SC in hemodynamic regulation, which should be considered in clinical setting and in research.
Similar content being viewed by others
Introduction
Excluding infrequent cases with anatomical variations, the human sympathetic innervation involves thoracic and not cervical spinal cord (SC) grey columns.1 The border between spinal cord injuries (SCIs) that cause prominent autonomic disturbances, such as autonomic hyper-reflexia, and that does not cause such disturbances is below the T5–T6 level rather than below the cervical SC,2 which implies that factors residing in the thoracic SC, such as nerve cells that affect muscular and splanchnic circulation, are necessary for intact cardiovascular regulation. Several publications show that cardiovascular impairments may be expressed differently in cervical and thoracic SCI, and that the autonomic deficit following thoracic SC lesions may have a specific clinical significance.3, 4, 5 Responses to liquid food ingestion, cold application to the hand and cold application to the foot6, 7, 8 indicated that the hemodynamic changes are mediated by a mid-thoracic SC mechanism.
But data showing a role for the mid-thoracic SC in the control of hemodynamic changes in the upright position has been scarce. The experiments presented in this study investigated the hypothesis that the mid-thoracic SC has such a role.
Materials and methods
Subjects
A total of 13 healthy control subjects and 21 persons with SCI of 3 months to 41 years duration were included in the study. Neurological levels of injury were determined using the International Standards for the Neurological Classification of Spinal Cord Injury and SCI severity was determined using the American Spinal Injury Association Impairment Scale (AIS).9 The 10 T4–T6 paraplegia and 11 C4–C7 tetraplegia SCI participants were patients who could be enrolled during the study period from the above T7 SCI patients admitted to the Spinal Department of Loewenstein Rehabilitation Hospital for rehabilitation or check-up. The control subjects were nine men and four women who were 34±13 years old. The persons with paraplegia were eight men and two women who were 38±13 years old, with AIS grade A SCI. Mean duration of their injury was 8.47 years. The persons with tetraplegia were 11 men, 42±8 years old, eight with AIS grade A and three with AIS grade B SCI. Patients with AIS grade B had hypoesthesia below the neurological level, whereas four of the patients with AIS grade A tetraplegia had anesthesia and four of them had hypoesthsia over 4–11 segments below the neurological level. Mean duration of injury in the patients with tetrapleia was 7.26 years. Participants did not present any medical conditions that could affect the results, such as febrile disease, heart failure, renal failure, diabetes mellitus or any additional neurological impairment, and were not receiving any medications that could affect testing.
Procedure
The Ethics Committee of Loewenstein Rehabilitation Hospital approved the study, and all participants signed an informed consent. In the morning, after a 12-h fast, each subject lay supine on an electric tilt-bed, in a relatively quiet hospital environment with an ambient temperature of about 22 °C. Subjects were tied to the bed with wide bands around the chest, pelvis and knees, avoiding pressure to the abdomen. A right cubital intravenous catheter was inserted and rinsed with diluted heparin. All subjects were continuously monitored for heart rate (HR), blood pressure (BP) and cerebral blood flow velocity (CBFV).
Participants were monitored for 30 min lying flat in a supine position, followed by 10 min with a 35 °C head-up tilt (HUT) and an additional 15 min rest, without tilt. Thereafter continuous monitoring and intravenous catheter were used for additional experiments, with the same patients and set-up, following the experiment described in this study. To detect a possible respiratory effect on measurements, the intravenous catheter was also used for venous blood partial pressure of carbon dioxide (PCO2) sampling in 10 control subjects, five persons with paraplegia and 10 persons with tetraplegia.
Recording and analysis
For HR recording, continuous electrocardiogram traces were obtained using surface electrodes and a preamplifier A/D system (Biopac Systems, Goleta, CA, USA). The Finapres device (Ohmeda Medical, Inverness, CO, USA) was applied for non-invasive continuous BP recording by means of a pressure cuff connected to the subjects’ middle finger. Electrocardiogram and the finger arterial pressure signals were simultaneously sampled online at 500 Hz. The digitized electrocardiogram signal was converted offline into a continuous beat-to-beat HR signal and resampled offline at 10 Hz. The digitized arterial pressure signal was low-pass filtered and resampled offline at 10 Hz.
For analysis in the time domain, we used the mean of HR and BP values resampled for each subject from the continuous signals. To compute the mean values of the arterial pressure at the heart level (BP) during HUT, hydrostatic pressure differences at 35 °C HUT between heart and finger were subtracted from the mean values of the finger arterial pressure. To obtain the hydrostatic pressure differences in mm Hg, we measured the distances from the finger to the middle of the arm, multiplied these by the sine of the tilt angle and divided the product by the specific gravity of mercury.
For analysis in the frequency domain, a spectral analysis of HR variations was performed after applying a median filter with a 251-sample length.10 Spectral analysis shows the sinusoidal components of the HR ultradian fluctuations and computes the power (or amplitude square) of each component. Plotting the power of BP and HR fluctuations against their frequency displays the typical peaks of the power around 0.15 and 0.4 Hz. The higher frequency fluctuations (HFs) correspond to the breathing cycle and are vagally mediated, as shown by elimination of this peak of the spectral plotting under total vagal blockade. The lower frequency fluctuations (LFs), presumably of vasomotor brainstem center and baroreceptor origin, are mediated by both sympathetic and parasympathetic tone and are affected by pharmacological blockade of either.10
A Discrete fourier transform was used in combination with the Welch Periodogram method to compute the power of the sampled HR signal fluctuations as a function of their frequency.11 The integrals of the power between 0 and 0.17 Hz and between 0.17 and 0.5 Hz were calculated to obtain HR LF and HF.
A transcranial Doppler (Smartlite—Rimed, Raanana, Israel) was used to record CBFV. The transcranial Doppler probe, applied to the subjects’ right temple, transmitted a 2 MHz pulsed wave through the ultrasonic window in the temporal bone. The Doppler frequency shift of the reflected wave was recorded by the device to compute the flow speed in a proximal segment of the middle cerebral artery. The CBFV signals were digitized online at 2 Hz and subjected to an off-line time-dependent analysis.
PCO2 was measured using the Stat Profile pHOx Analyzer (Nova Biomedical, 200 Prospect Street Waltham, MA, 02453 USA).
Statistical analysis
The mean values of HR, BP, HR LF and HF, the ratio HR LF/HF and mean CBFV (mCBFV) were calculated at supine rest (during 5 min before the tilt for the time domain, and during 10 min for the frequency domain) and during HUT. Findings during rest and HUT were compared using analysis of variance with repeated measurements within and between groups (paraplegia, tetraplegia and healthy participants), to examine the HUT effects and the effects of the groups themselves on the hemodynamic-dependent variables. Post hoc comparisons between groups and conditions were performed to determine the specific effect of SCI on the hemodynamic variables. Correlations between changes in variables were examined using Pearson's correlation test. Before the statistical analysis, the spectral components were subjected to a square root transformation and their ratios to a natural logarithm transformation to approach normal distributions. Data were analyzed by SPSS for Windows, version 11 (SPSS, Chicago, IL, USA).
Results
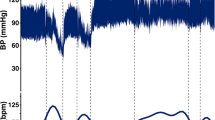
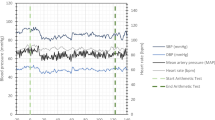
The differences in age and SCI duration between the groups were non-significant (P>0.2). Most of the subjects completed the tilt testing without clinical disturbances, except two persons with tetraplegia who felt unwell during HUT, and their BP and CBFV measurements for 5–10 min of HUT could not be included in the analysis. Hemodynamic variables at supine rest, and responses to HUT in the time and frequency domains are illustrated in Figures 1 and 2. Their mean and s.d.. values are detailed in Table 1, and the main effects of HUT on hemodynamic variables are summarized in Table 2.
HR spectral power (square amplitude of HR fluctuations at each frequency). Top table: HR power in the control, paraplegia or tetraplegia groups before HUT. Lower table: HR power in the control, paraplegia or tetraplegia groups during HUT. Lower (darker) curve: HR power average values. Upper (lighter) curve: HR power average+s.d. values.
Hemodynamic variables in the time domain
Group effect on HR and mCBFV was not significant, but on BP it was significant (P<0.01) during supine rest and HUT: in the tetraplegia group BP was lower than in the other subject groups.
HUT effect on HR and mCBFV was significant (P<0.001) irrespective of group (P<0.05): it increased HR and decreased mCBFV. The decrease in mCBFV was slight in the control and paraplegia groups and marked in the tetraplegia group. HUT effect on BP, however, was different in various groups (P<0.02): mean BP during HUT was slightly higher than that during supine rest in the control and paraplegia groups, but markedly lower in the tetraplegia group.
Hemodynamic variables in the frequency domain
Group effect was significant (P<0.05) on the LF and the HF HR spectral component, but not on natural logarithm HR LF/HF. HR LF and HF were higher in healthy subjects than in SCI individuals, and lowest in persons with tetraplegia at supine rest and HUT.
HUT effect on the LF or HF HR variation spectral analysis components was not significant, but HUT had a significant effect on HR LF/HF (P<0.01): natural logarithm HR LF/HF increased at HUT similarly in all groups. However, the group affected the correlation between increases in HR LF/HF and HR: natural logarithm LF/HF increase was correlated with HR increase in the control group (r=0.72; P<0.01) and in the tetraplegia group (r=0.77; P<0.01), but not significantly in the paraplegia group (r=0.63; P>0.09).
HUT effect on PCO2
Venous PCO2 at supine rest was 51.8±7.9 mm Hg−1 in the control group, 54.1±2.6 in the paraplegia group and 46.91±6.7 in the tetraplegia group. Around 5 min after tilting it was 48.5±7.5 in the control group, 50.2±4.6 in the paraplegia group and 45.4±6.6 in the tetraplegia group. The differences between groups and HUT effect on the measured PCO2 were not statistically significant.
Discussion
The results of this experiment are generally compatible with previously published data.5, 12, 13, 14, 15, 16, 17, 18 We did not find, however, publications of HUT studies with series of T4–T6 paraplegia patients for comparison. Therefore, we consider the findings in this specific group to be novel and noteworthy.
Two findings support the study hypothesis: (a) the absence of a significant BP decrease, and even some BP increase in T4–T6 paraplegia, in response to HUT; and (b) the significant correlation between HR increase and HR LF/HF increase, at HUT, in the control and tetraplegia groups, but not in the paraplegia group.
It may be argued that in the present experiment BP was maintained at HUT in T4–T6 paraplegia, but not in tetraplegia, by efferent signals of the baro-reflex conveyed to the heart and blood vessels through intact segments above T5. But this is unlikely because in another experiment these segments were not sufficient to compensate for a drop in BP, smaller than the drop in tetraplegia in this study, in the same T4–T6 paraplegia group following a meal.6 This indicates that the baro-reflex alone is not likely to maintain BP and that another mechanism is maintaining BP in T4–T6 paraplegia at HUT. This mechanism may be maintaining BP by damage to a hypothetic mid-thoracic neural structure that induces vasodilatation and enhances hypotension at HUT, but not after meals. The mechanism would maintain BP in control and paraplegia patients, but not in tetraplegia, because the activity of the mid-thoracic SC neural structure, which is reduced by SCI in mid-thoracic paraplegia, is suppressed in healthy subjects, through pathways descending through the cervical SC, but is not affected in patients with tetraplegia whose cervical SC is damaged.
The mid-thoracic SC neural structure may respond to afferent nerve fibers reacting to increased tension during HUT in splanchnic and lower body vessel walls. It may include a signal generator transmitting efferent stimuli to the heart through the sympathetic ganglia chain without passing through the cervical SC. Generation of such stimuli, which affect HR or HR variation depending on the integrity of the T4–T6 thoracic and not necessarily of the cervical SC, can explain the significant correlation between HR increase and HR LF/HF increase in the control and tetraplegia groups, but not in the paraplegia group.
Additional findings show that despite the non-significant change in BP at HUT in the control and paraplegia groups, HR increased and CBFV decreased. These can be reactions to arterial vasoconstriction above the neck, as part of compensatory baroreflex that participates in maintaining BP at HUT in these groups. A decrease in CBF, reflected by significant CBFV decrease in the control and paraplegia groups, may contribute to the sympatho-vagal balance increase during HUT in these groups, which is represented by the significant HR LF/HF increase. These findings are compatible with involvement of the thoracic SC in hemodynamic regulation.
Implications
The findings support the study hypothesis and add information to be considered when assessing patients with thoracic SCI in clinical setting and in research. For example: (a) orthostatic hypotension in a person with chronic T4–T6 paraplegia should raise suspicion of an additional lesion because of the observed mild or transient nature of BP response to HUT in T4–T6 SCI patients; (b) if the observation is confirmed, orthostatic hypotension in these patients, which is usually mild if present, may be treated with non-pharmacological measures, avoiding at least part of the measures recommended by the European Federation of Neurological Societies (EFNS) guidelines for the management of orthostatic hypotension;19 (c) the mid-thoracic neural mechanism suggested by this study indirectly supports the minimal autonomic hyper-reflexia expression in patients with T4–T6 SCI, demonstrated in another publication.8
Limitations
Limitations of the study include an absence of anatomically complete lesions, multiple analyses, a relatively small study population, a possible effect of respiration during HUT on findings and possible minor errors in the non-invasive BP measurement.
The use of clinical criteria for enrollment and the multiple analyses were discussed in our previous publications,6, 7, 8 and are unlikely to have affected the results.
More subjects in each category would have added power to the study, but the availability of patients who fit the inclusion criteria and could be enrolled during the study period was limited.
Changes in breathing and PCO2 could have affected measurements, mainly CBFV, during HUT, but venous blood PCO2 sampling showed non-significant differences between supine rest and mid-HUT in all three groups. Moreover, HR HF variations, which are affected by respiratory changes, did not show a significant change at HUT. Respiratory changes are, therefore, unlikely to have had a remarkable effect on the findings.
The fact that nearly syncopal BP measurements of two SCI individuals could not be included in the analysis has affected the average BP, but were these measurements included, the demonstrated HUT effect on HR and BP would be even stronger. At the same time, the method used to calculate the arterial pressure at heart level introduced a measurement error during the short period of transition from supine rest to HUT. These biases, however, were minor, had opposite tendencies and are not expected to affect the inferences.
A typical bias that the Finapres device may introduce, in the order of 2–4 mm Hg,20 not always in the same direction, is unlikely to affect the results because errors with different directions may cancel each other out within and among groups, and because assessing HUT effect on BP by comparing changes in BP and not absolute BP values, minimized this effect.
Conclusions
Our results were generally consistent with those of comparable studies, but showed, in addition, a remarkable discrepancy between persons with tetraplegia, who responded with a significant BP decrease to HUT, and persons with T4–T6 paraplegia, who did not. This finding, and additional (less prominent) ones support a role for the mid-thoracic SC in BP and HR regulation, and suggest that a neural signal generator in the thoracic SC transmits stimuli to the heart, independent of the cervical SC. The suggested role of the mid-thoracic SC adds information to be considered in clinical setting and in research.
References
Appenzeller O, Oribe E . Autonomic anatomy histology and neurotransmission. In: Appenzeller O, Oribe E (eds). The Autonomic Nervous System: An Introduction to Basic and Clinical Concepts. Elsevier: Amsterdam, 1997, pp 4.
Mathias CJ, Frankel HL . Autonomic disturbances in spinal cord lesions. In: Mathias CJ, Bannister R (eds). Autonomic Failure. Oxford University Press: Oxford, 1999, pp 494–513.
Baliga RR, Catz A, Watson LD, Short DJ, Frankel HL, Mathias CJ . Cardiovascular and hormonal responses to food ingestion in humans with spinal cord transection. Clin Autonom Res 1997; 7: 137–141.
Catz A, Mendelson L, Solzi P . Symptomatic postprandial hypotension in high paraplegia. Case report. Paraplegia 1992; 30: 582–586.
Aslan SC, Randall DC, Donohue KD, Knapp CF, Patwardhan AR, McDowell SM et al. Blood pressure regulation in neurally intact human vs. acutely injured paraplegic and tetraplegic patients during passive tilt. Am J Physiol Regul Integr Comp Physiol 2007; 292: R1146–R1157.
Catz A, Bluvshtein V, Pinhas I, Akselrod S, Gelernter I, Nissel T et al. Hemodynamic effects of liquid food ingestion in mid-thoracic paraplegia: is postprandial hypotension related to thoracic spinal cord damage? Spinal Cord 2007; 45: 96–103.
Catz A, Bluvshtein V, Pinhas I, Akselrod S, Gelernter I, Nissel T et al. Cold pressor test in tetraplegia and paraplegia suggests an independent role of the thoracic spinal cord in the hemodynamic responses to cold. Spinal Cord 2008; 46: 33–38.
Catz A, Bluvshtein V, Korczyn AD, Pinhas I, Gelernter I, Nissel T et al. Modified cold pressor test by cold application to the foot after spinal cord injury: suggestion of hemodynamic control by the spinal cord. American Journal of PM&R 2007; 86: 875–882.
Maynard Jr FM, Bracken MB, Creasey G, Ditunno Jr JF, Donovan WH, Ducker TB et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997; 35: 266–274.
Akselrod S . Spectral analysis of fluctuations in heart rate and other cardiovascular parameters as a tool for assessment of autonomic control. In: Korczyn AD (ed). Handbook of Autonomic Nervous System Dysfunction. Marcel Decker: New York, 1995, pp 469–493.
Keselbrener L, Akselrod S . Selective discrete Fourier transform algorithm for time-frequency analysis: method and application on simulated and cardiovascular signals. IEEE Trans Biomedical Eng 1996; 43: 789–802.
Edwards MR, Shoemaker JK, Hughson RL . Dynamic modulation of cerebrovascular resistance as an index of autoregulation under tilt and controlled PETCO2 . Am J Physiol Regul Integr Comp Physiol 2002; 283: R653–R662.
Inoue K, Ogata H, Hayano J, Miyake S, Kamada T, Kuno M et al. Assessment of autonomic function in traumatic quadriplegic and paraplegic patients by spectral analysis of heart rate variability. J Auton Nerv Syst 1995; 54: 225–234.
Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelly RE . Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia 1991; 29: 1–7.
Laszlo Z, Rosler A, Hinhhofer-Szalkay HG . Cardiovascular and hormonal changes with different angles of head-up tilt in man. Physiological Research 2001; 50: 71–82.
Mathias CJ, Bannister R . Investigation of autonomic disorders. In: Mathias CJ, Bannister R (eds). Autonomic Failure. Oxford University Press: Oxford, 1999, pp 169–195.
Wieling W, Karemaker M . Measurements of heart rate and blood pressure to evaluate disturbances in neurocardiovascular control. In: Mathias CJ, Bannister R (eds). Autonomic Failure. Oxford University Press: Oxford, 1999, pp 196–210.
Butler GC, Yamamoto Y, Hughson RL . Heart rate variability to monitor autonomic nervous system activity during orthostatic stress. J Clin Pharmacol 1994; 34: 558–562.
Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M . EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 2006; 13: 930–936.
Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G . Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 1989; 13: 647–655.
Acknowledgements
This study was supported by the Unit of Medical Services, Rehabilitation Department, Ministry of Defense and by the Tel-Aviv University Research Fund. Rimed Ltd., Raanana, Israel lent the TCD device for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bluvshtein, V., Korczyn, A., Akselrod, S. et al. Hemodynamic responses to head-up tilt after spinal cord injury support a role for the mid-thoracic spinal cord in cardiovascular regulation. Spinal Cord 49, 251–256 (2011). https://doi.org/10.1038/sc.2010.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.98
Keywords
This article is cited by
-
Insulin resistance in tetraplegia but not in mid-thoracic paraplegia: is the mid-thoracic spinal cord involved in glucose regulation?
Spinal Cord (2011)
-
Response to Cardiovascular control during head-up tilt test in spinal cord injury patients
Spinal Cord (2011)
-
Cardiovascular control during head-up tilt test in spinal cord injury patients
Spinal Cord (2011)