Abstract
Study design:
All randomized controlled trials, prospective cohort, case–controlled, pre–post studies and case reports that assessed exercise interventions, which influence arterial structure and function after spinal cord injury (SCI), were included.
Objective:
To review systematically the evidence for exercise as a therapy to alter arterial function in persons with SCI.
Setting:
Literature searches were conducted for appropriate articles using several electronic databases (e.g. MEDLINE, EMBASE).
Methods:
Three independent reviewers evaluated each investigation's quality, using the Physiotherapy Evidence Database Scale for randomized controlled trials and Downs and Black Scale for all other studies. Results were tabulated and levels of evidence assigned.
Results:
A total of 283 studies were found through the systematic literature search. Upon review of the articles, 27 were included. The articles were separated into those investigating arterial benefits, resulting from either acute bouts of exercise or long-term exercise interventions. The ability of both acute and long-term exercise interventions to improve arterial structure and function in those with SCI was supported by limited to moderate methodological quality. Upper body wheeling is the most commonly examined exercise therapy for improving arterial function. It appears from the evidence that a variety of exercise interventions, including passive exercise, upper body wheeling, functional electrical stimulation and electrically stimulated resistance exercise, can improve arterial function in those living with SCI.
Conclusions:
Although the quality and volume of evidence is low, the literature supports exercise as a useful intervention technique for improving arterial function in those with SCI.
Similar content being viewed by others
Introduction
Cardiovascular disease is a leading cause of death in those with spinal cord injury (SCI).1, 2, 3 Increased vascular stiffness is thought to cause an amplified pulse pressure, a reduction in pressure buffering,4 propagate increased ventricular tension and is a primary marker of cardiovascular disease risk.5, 6 Increasing vascular stiffness is related to advancing age,7, 8 poor nutrition,9, 10 smoking,11, 12 excessive alcohol consumption,2, 13, 14 other chronic conditions (such as heart disease)15 and low levels of physical activity.16 Owing to the lack of mobility and the loss of autonomic regulation, people with SCI are particularly susceptible to alterations in both central and peripheral vascular function.17, 18, 19, 20 Moreover, it has been shown recently that those with SCI have increased vascular stiffness when compared with able-bodied (AB) individuals.21, 22
Shortly after an SCI, femoral artery diameter and leg blood flow decrease significantly,23 eventually by up to 50% in comparison to AB individuals.24 It is thought that the loss of voluntary muscle contraction below the lesion level reduces blood flow to the legs: due to both a lack of oxygen demand and reduced release of metabolically stimulated vasodilatory signals (carbon dioxide, potassium, hydron, adenosine, and so on).25 Another potential mechanism is a loss of the physical lengthening and shortening of muscle tissue, which inhibits the muscle pump and reduces the arterio-venous pressure gradient across the muscle bed.26, 27, 28 Finally, in response to inactive muscular tissue and chronically reduced blood flow demand, the arterial system reacts by reducing the diameter29 and the capillary density,30 causing an increase in vascular resistance and leading to decreased venous capacitance and compliance.31
The loss of autonomic control below the lesion level appears to influence vascular stiffness as well. Although sympathetic denervation would be expected to increase vascular elasticity owing to a reduction in vascular tone, it appears that this occurs only in a transient nature,32 and with long-term autonomic denervation, affected vessels increase stiffness as compared with pre-injury levels.33 Supporting this idea is evidence showing that reduced autonomic presence in denervated limbs increases expression of endothelin-1 and reduces expression of nitric oxide, which would lead to increased arterial stiffness and resistance.34
It is well established that physical exercise generally improves cardiovascular health35, 36 and has been shown to improve arterial function in a variety of populations, disorders and disease states.7, 36, 37, 38, 39 Several papers have been published on the influence exercise has on cardiovascular function in those with SCI (see review of Warburton et al.36). The purpose of this systematic review is to present a synopsis of the scientific literature investigating the usefulness of various exercise strategies to improve vascular function in denervated limbs of those with SCI. We hypothesize that there will be strong support for the therapeutic vascular benefits of exercise training in persons living with SCI. Furthermore, we expect that exercise modalities, which employ electrical stimulation of denervated muscle, will have stronger support as compared with techniques, which allow passive motion of denervated limbs.
Methods
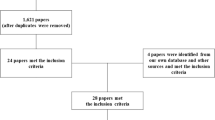
A keyword literature search for all scientific publications from 1950 to present investigating the influence of exercise on vascular stiffness was conducted using the following online databases: MEDLINE, EMBASE, Cochrane Library, ACP Journal Club, DARE, CCTR, CMR, HTA, NHSEED, PsycINFO, SPORTDiscus and CINAHL. Population key words—spinal cord injuries, spinal cord injury, paraplegia, tetraplegia and quadriplegia—and vascular function keywords—vascular stiffness, vascular compliance, vascular function, vascular elasticity, vascular resistance, arterial stiffness, arterial compliance, arterial resistance, arterial function, arterial elasticity, endothelial function, artery stiffness, artery compliance, artery function, artery elasticity, blood flow, arterial flow, leg blood flow, femoral artery flow and femoral blood flow—as well as exercise phrases—exertion, exercise, movement, locomotion, running, jogging, swimming, walking, dependent ambulation, motor activity, muscle contraction, isometric contraction, isotonic contraction, weight lifting, exercise therapy, physical, physical activity, body weight supported treadmill training (BWSTT) and functional electrical stimulation (FES)—were paired by permutation. A total of 283 papers were found, after which duplicates, review papers, letters to the editor, those not in English and those not evaluating arterial function outcomes were removed from the sample leaving a total of 26 articles (Figure 1). Two additional papers40, 41 were added to the sample as a result of cross-referencing, leaving a total of 28 articles.
An evaluation of the methodological quality of each article was completed by two independent reviewers (AP, AC) and confirmed by a third reviewer with expertise in systematic reviews (DW) using either the 11-item PEDro Scale42 (randomized controlled trials; RCT) or the Down and Black Tool43 (non-RCTs). The highest and therefore most methodologically strong score attainable for a given research article is 10 for the PEDro Scale and 27 for the Down and Black Tool. Higher points indicate a superior methodological quality. Further, the level of evidence was evaluated using a five-level scale43 (simplified form of Sackett),44 where level 1 (the highest level of evidence)=RCT with a PEDro score ⩾6; level 2=an RCT with a PEDro score ⩽5, a non-randomized prospective-controlled study, or a cohort study; level 3=a case–control study; level 4=a pre- and post-test or a case series; and level 5 (the lowest level of evidence)=an observational report or case report with only a single subject.45 There was no minimum sample size owing to the low number of publications in SCI research. Librarians from the University of British Columbia and all authors approved this systematic process and methodology. Ranking scores were performed in duplicate, after which any discrepancies were solved by discussion.
Results
The articles selected for investigation were categorized into two groups: Acute Exercise and Non-Acute Exercise. Within the Acute Exercise group, the Down and Black Tool scores ranged from 10 to 19 out of 27 (limited to moderate methodological strength).46, 47, 48 The lone RCT49 categorized within the Non-Acute Exercise group received a PEDro Scale score of six out of 10 (good methodological quality),50 whereas the remaining studies ranged from 10 to 20 out of 27 on the Down and Black Scale (considered limited to moderate methodological quality).46
Acute exercise techniques
Fourteen papers investigated the vascular effects of a single acute exercise bout. Eight articles were prospective control trials31, 41, 51, 52, 53, 54, 55, 56 and six were pre–post design.40, 57, 58, 59, 60, 61 Papers investigating acute exercise included: passive leg exercise (n=3),53, 57, 60 FES (n=3),54, 55, 59 single muscle electrical stimulation (n=1),56 upper body continuous aerobic exercise (arm cycling or wheeling) (n=5),31, 40, 41, 52, 58 acute combined arm passive leg exercise (n=1)61 and stretch-induced contractions (n=1).51 Tables 1 and 2 are a summary of published investigations researching the effect of acute exercise on arterial function in SCI individuals.
Acute passive leg exercise
Passive leg exercise, or the application of external forces on denervated limbs with the purpose of causing motion, is the simplest technique for lower body exercise in the SCI population.36 Three papers investigated the vascular effect of passive exercise on arterial function in SCI participants. One examined the influence of passive range of motion physiotherapy on leg blood flow in paraplegics (PARA) and tetraplegics (TETRA).60 This article showed that leg blood flow increases very little, if at all, in response to passive leg movement administered by a physiotherapist.60 The other two studies from this group investigated passive cycling (denervated legs are strapped to pedals and pedals are mechanically rotated) and showed disagreement in their results. Ter Woerds et al.53 measured arterial function before, during and after 10 min of passive leg movements, and 20 min of passive leg cycling for PARA and AB individuals. These authors showed no change in femoral blood flow or leg vascular resistance (calculated as mean arterial pressure divided by leg blood flow) for either group during or after (0, 1, 2, 5 or 10 min post-exercise) either of the passive exercise modalities. In contrast, Ballaz et al.57 reported that leg blood flow (measured using Doppler ultrasound) increased in PARA after 10 min of passive cycling. These authors acknowledged the discrepancy and inferred a number of methodological differences between studies to account for marked disparities (such as higher cadence, continuous exercise without 10-s rest periods for flow measurements, larger sample size and optimal force arm length to involve the maximum musculature for a given pedal rotation).57
Conclusions: There is level 4 evidence (indicating available evidence but without comparable groups) that a single bout of passive leg exercise increases leg blood flow,57 whereas there is level 253 (debatable but reliable results) and level 4 evidence60 suggesting no change in leg blood flow or leg vascular resistance in response to passive leg exercise. As only three papers have published results on leg blood flow changes in response to passive leg exercise, further research, incorporating various exercise intensities and force arm lengths with adequate sample sizes, is warranted.
Acute FES exercise
FES involves muscle stimulators placed over motor points in denervated musculature activated in a synchronized manner as to functionally manipulate an exercise device (e.g. a cycle ergometer). FES is one of the most widely studied rehabilitation techniques for the SCI population. The application of FES has shown promise for a variety of SCI-related issues including obesity62 and muscle atrophy.63 A total of three papers have investigated acute arterial function changes in response to FES.54, 55, 59 Nash et al.55 showed similar ankle/brachial index (the ratio of blood pressure in the lower legs to the blood pressure in the arms) at rest between age- and gender-matched AB and TETRA men. After an acute 30-min bout of FES, however, ankle/brachial index in AB men increased with no change in TETRA. These findings were explained by increased lower body blood pooling after exercise or decreased vascular response post-exercise in TETRA.55
The remaining two papers measuring arterial function in response to acute bouts of FES showed increased leg blood flow after exercise. In the investigation by Phillips et al.,59 pre–post exercise changes in toe photoelectric plethysmography were used to estimate changes in leg blood flow in SCI participants (C6–T12). The participants performed two different FES intensities (40 mA, 80 mA) in conjunction with arm crank exercise (hybrid), and adjusted resistance to create an intensity of 60 or 80% of VO2 peak (four trials total). These authors reported significantly elevated leg blood flow after hybrid exercise and increasing blood flow with increasing hybrid exercise intensity, suggesting that leg blood flow in SCI is intensity dependent.59 Dela et al.54 also showed increased leg blood flow with FES and no difference in leg blood flow or leg oxygen uptake between TETRA, PARA or AB after 15 min of low-intensity FES. However, for those with SCI, these variables leveled off and did not increase at the higher FES intensity as was noted in the AB group.
Conclusions: There is level 2 evidence54, 55 (reliable results but debatable) suggesting that FES exercise can improve arterial function in those with SCI. Studies examining acute bouts of FES illustrate its usefulness in identifying vascular changes in those unable to willfully contract musculature. The findings of the three studies investigating SCI arterial function in response to FES show that lower body exercise of this modality can cause an increase in leg blood flow in both TETRA and PARA. It appears that further investigations involving a variety of intensities and durations of FES are needed to clarify disagreement in the dose–response relationship between exercise and arterial dynamics in SCI. In addition, to maximize potential arterial benefits, different modalities (specifically concurrent arm cranking (hybrid) vs resting upper body) need to be compared.
Acute single muscle electrical stimulation
One article investigated the influence of single muscle electrical stimulation on arterial dynamics in those with SCI.56 This study illustrated that electrical stimulation of the quadriceps femoris leads to increased femoral artery blood flow. In addition, these authors showed that femoral blood flow increases with increasing exercise intensity in SCI participants, suggesting a dose–response relationship.
Conclusions: Currently, there is level 4 (available evidence but without comparable groups) evidence suggesting that single muscle leg electrical stimulation improves femoral artery blood flow in those with SCI. Only one study has investigated this type of intervention (n=17, 9 SCI, 8 AB); therefore, there is a great need to examine further the effect of single muscle electrical stimulation on arterial dynamics in those with SCI.
Acute arm exercise
Upper body exercise for individuals with SCI is a relatively inexpensive and simple technique to increase physical activity. The arterial changes in SCI persons induced by acute bouts of upper body exercise have been studied in five31, 40, 41, 52, 58 articles. Three papers from this group investigated blood flow changes in response to arm cranking.40, 41, 52 The common finding from this group of investigations is that the ability of the denervated vasculature to locally regulate blood volume and redistribute it to working muscles (i.e. the upper body during arm cycling) is impaired. Bidart and Maury52 showed no change in leg blood volume (plethysmography) during 1 min of arm cranking in SCI, whereas leg blood volume decreased substantially during the same exercise in AB. Kinzer and Convertino40 showed that leg blood volume increases after 10 min of arm cycling in SCI, but decreased in AB. Hopman et al.41 illustrated convincingly the impaired local regulation in denervated lower limbs by showing that both the rate of leg blood volume changes and the absolute level of leg blood volume were significantly reduced in SCI as compared with an AB control group during 25 min of arm cycling.
In addition to investigating leg blood volume changes, leg blood flow was also measured during upper body cycling exercise in four of the five papers.31, 40, 52, 58 Kinzer and Convertino40 as well as Bidart and Maury52 and Burkett et al.58 showed increases in leg blood flow during upper body arm cycling at moderate40, 52 and maximal58 intensity levels (durations of 4–15 min). The results of Kinzer and Convertino40 as well as Burkett et al.,58 however, failed to show significant changes, and work by Bidart and Maury52 did not include a statistical analysis or original data. Hopman et al.31 took this line of research a step further and illustrated that there is no statistically significant increase in femoral artery blood flow during 25 min of upper body cycling. The data from this paper, however, does show a trend of increasing mean femoral artery blood flow velocity, with no change in arterial diameter (flow=velocity × diameter).31
Conclusions: There is level 2 evidence40, 41, 52 (reliable results but debatable) showing that local blood volume regulation in denervated limbs is impaired during upper body cycling in SCI. In addition, there is level 2 evidence40, 52, 58 (reliable results but debatable) suggesting that leg blood flow increases during upper body cycling; however, these conclusions were not verified statistically. There is however level 2 evidence showing that leg blood flow does not increase to a statically significant level during upper body cycling exercise.31 More research is needed to clarify the minimum duration and intensity of arm exercise required to improve leg blood flow. Also, more research is required with regard to changes in leg blood flow during upper body cycling with larger sample sizes and relevant statistical analyses.31
Acute combined arm passive leg exercise
Only one study has examined arterial function after a single bout of active arm exercise combined with passive leg movement.61 This pre–post investigation showed that blood flow measured by double isotope disappearance rate increased in the tibialis anterior after simultaneous 30 repetition bouts of arm movement against resistance and passive leg movement performed by a physiotherapist.
Conclusions: There is currently one paper with level 461 evidence (available evidence but without comparable groups) that reported increased leg blood flow in response to combined arm exercise and passive leg movements. With such limited data, it is difficult to interpret the value of this exercise technique. Further research is required in this area.
Stretch-induced contractions
One study has investigated the changes in arterial structure and function in response to stretch-induced contractions of denervated limbs in those with SCI.51 The investigation by Bidart and Maury, which describes manually stretching the gastrocnemius to induce a spasmodic reflex contraction, found similar local blood flow responses in the gastrocnemius after stretch-induced contractions in AB and PARA. The authors also noted that because there was no quantitative comparison of the strength of spasmodic manual stretch reflex to voluntary contraction of the AB group, quantitatively comparing the blood flow response is speculative.
Conclusions: There is currently one paper with level 2 (debatable but reliable results) evidence investigating blood flow changes in response to stretch-induced contractions.51 With such limited data, it is difficult to interpret the value of this exercise technique. More research needs to be completed investigating64, 65, 66, 67, 68 the acute vascular changes in response to stretch-induced contractions.
Exercise training interventions
Fifteen papers met the systematic search criteria that examined changes in arterial function and structure resulting from exercise training (i.e. non-acute exercise) interventions. Two articles were case–control studies,69, 70 11 were of the pre–post design,24, 71, 72, 73, 74, 75 one was a case report and one was an RCT. The Non-Acute Exercise category (n=15) included passive leg exercise (n=1),49 FES (n=5),65, 70, 71, 72, 73 upper body exercise (n=2),69, 76 an electrically stimulated resistance training regimen (n=3),24, 67, 68 hybrid exercise (n=3)66, 74, 75 and BWSTT (n=1).64 Table 3 is a summary of published investigations examining the effect of non-acute exercise on arterial function in those with SCI.
Passive leg exercise
Only one study investigated the effect of a passive, non-acute exercise program on arterial function in PARA.49 This study showed that an un-supervised 6-week home-based passive cycling intervention improves femoral artery hemodynamic response (ultra-sound derived femoral blood flow velocity) in those with SCI (n=9) after 10-min passive cycling exercise (P=0.01), but does not change resting femoral artery blood flow (P=0.08). The control group (n=8), which continued their habitual daily routine, showed no change in either measure.
Conclusions: There is currently level 1 evidence49 (highly reliable) supporting a passive leg exercise program as a technique to improve vascular function in PARA. Although only one study has investigated this intervention technique, the high level of evidence suggests that routine passive exercise improves lower body vascular function in PARA. There is a need to investigate long-term passive cycling exercise programs with larger sample sizes that include TETRA.
FES
Several investigations have examined the effect of non-acute FES training for improving arterial dynamics in SCI. All five papers investigating FES cycling showed significant improvements in vascular function in both PARA and TETRA. Overall, FES has led to increased femoral artery blood flow,70, 71, 73 improved small artery compliance,65 femoral artery compliance,72 normalized femoral flow-mediated dilatation,72 reduced leg vascular resistance73 and improved hyperemic response.70 According to work by De Groot et al.,23 a minimum of 2 weeks FES training is required for arterial dynamic improvements (femoral artery flow-mediated dilatation); however, trends start to appear after the first week. After 4 weeks of FES training, femoral arterial compliance and femoral artery blood flow showed statistically significant improvements.
Conclusions: Based on the available evidence, including a level 3 (ref. 70) (somewhat reliable) investigation, there is promising support for the use of long-term FES training in improving vascular health in SCI.
Arm exercise
Two papers have evaluated the effect of non-acute upper body (arm) exercise on arterial function in SCI participants. Jae et al.69 compared intima–media thickness, compliance and β stiffness index (a measure of arterial elasticity) of the common carotid artery between 28 PARA competitive athletes and 24 age-matched recreationally active AB controls finding no differences between groups. In addition, Tordi et al.76 published a case study examining the aortic pulse wave velocity values of a single SCI participant before and after 6 weeks of upper body endurance training (30 min, 3 times per week) showing significant improvements in central aortic stiffness post-training.
Conclusions: These studies appear to suggest that long-term upper body exercise can improve arterial structure and function in those with SCI. The study design (level 369 and level 576 evidence (somewhat reliable–to reliable)) of these investigations illustrates the need for prospective research examining the influence of non-acute upper body exercise on arterial dynamics in SCI participants with larger sample sizes and proper controls.
Electrically stimulated knee extension
Electrically stimulated knee extension, which involves electrical stimulation of the denervated musculature to increase strength, has been investigated by three studies as a potential technique for improving arterial structure and function in those with SCI.24, 67, 68 Two studies’ training regimen consisted of exercise 2 days per week, for 8 weeks. In the first study, improved quadriceps strength was found, but no improvements in femoral arterial structure or function (artery diameter, hyperemic response, exercise blood flow response).68 In the follow-up study from the same group, improved femoral artery flow-mediated dilatation was elevated after the training regimen.67 Another study performed by Taylor et al.,24 which used a progressive 3-month long knee extension exercise with the aim of allowing standing, illustrated increased muscle thickness and thigh blood flow.
Conclusions: It appears from level 4 evidence24, 67, 68 (available evidence but without comparable groups) that electrically stimulated knee extension increases quadriceps strength and improves some measures of lower body arterial function in those with SCI. More research is needed with SCI controls to verify the potential benefits of this therapy.
Hybrid exercise
A total of three studies investigated the effects of a long-term hybrid training intervention on arterial dynamics in those with SCI. Of those studies, two used FES cycling of the legs with voluntary arm cranking,74, 75 whereas the third study used a modified approach, which employed FES at the legs to assist with walking, while simultaneously supporting ones’ self using a walker to provide semi-autonomous ambulation.66 These studies reported improvements in femoral vascular function and structure for both TETRA74 and PARA participants,66, 75 including increased baseline femoral artery blood flow,74, 75 cross-sectional area,66 peak blood flow,75 diameter,74 flow-mediated dilatation74 and post-occlusion hyperemic response.66, 75 The dependency of these improvements on the exercise intervention is highlighted by Thijssen et al.,74 who showed that improvements occur after only 2 weeks of hybrid training and all vascular changes (with the exception of flow-mediated dilatation) disappear after just 1 week of de-training.
Conclusions: Hybrid exercise is a useful tool for improving vascular function in those with SCI. As of yet, no investigation has compared FES with hybrid exercise to identify whether hybrid exercise yields more vascular function gains than traditional FES training, particularly considering the increased cost of hybrid exercise equipment. The low level of evidence66, 74, 75 (level 4—limited reliability) for literature investigating the effect of long-term FES exercise interventions on arterial function and structure highlights the need for valid control groups.
Body weight-assisted treadmill training
Only one study examined the arterial function changes resulting from a non-acute program of BWSTT. Ditor et al.64 showed 4 months of training 3 days per week using BWSTT improved femoral arterial compliance, but did not increase femoral artery blood flow or carotid vascular compliance in those with TETRA or PARA.
Conclusions: It appears from this level 4 evidence64 (available evidence but without comparable groups), which showed that femoral artery compliance was increased, that BWSTT has the potential to improve vascular function in SCI participants. More studies are needed, however, with relevant (i.e. those with SCI) control subjects using varying intensities, protocols and levels of injury to clarify the maximum and minimum potential benefit, as well as timeline of functional gains for a given population.
Discussion
The purpose of this review was to compile and evaluate relevant literature examining the effect of various modes of exercise on arterial dynamics in those with SCI. We also separated articles into those investigating the effects of acute bouts of exercise and others investigating vascular changes from a multiple-session (non-acute) training intervention. As access to patients with low incidence disabilities such as SCI is very limited77 and the level of participation in physical activity within this population is lower than that of the AB population,78, 79 there is hesitation from researchers to design studies where half the willing and available participants would receive no intervention. Therefore, most research designs investigating SCI and exercise or physical activity involve poorly matched controls if present at all (level 2–5 evidence46). Testing the experimental group before and after a control phase, once the exercise intervention has been completed, may be a viable alternative, which would improve the level of evidence for studies limited in sample size.
Taking into consideration the lack of valid controls (i.e. those with SCI), the evidence still supports exercise as a viable method of improving vascular function in those with SCI. Following that, based on the available evidence, FES and hybrid exercise are the most promising types of exercise for improving arterial dynamics as they have been shown to increase femoral artery blood flow, reduce arterial stiffness and improve hyperemic response.65, 71, 72, 73, 74, 75 Finally, according to the available literature, to increase vascular health in those with SCI, hybrid or FES training with increasing levels of intensity should be performed a minimum of two times per week and continued indefinitely. Improvements in arterial structure and function begin to appear as early as 1 week into training and after 8 weeks show a range of arterial health benefits.
It should be noted that there is significant disagreement with regard to the ability of passive leg exercise to improve denervated arterial function, specifically femoral artery blood flow.49, 53, 57 In a published Letter to the editor,80 Hopman's group, which had shown no increase in femoral artery blood flow in response to passive leg exercise, questioned the results and methodology of work published by Ballaz et al.57 showing opposite results. Although no definitive conclusion resulted from this discussion, the influence of the muscle pump at increasing femoral artery flow in response to passive exercise was implicated as the potential mechanism. Research from our laboratory supports this concept and has shown the cardiac output to increase during passive exercise in patients with SCI.81 Further, others have shown the muscle pump to play a relatively small but significant role in leg blood flow during passive movement in AB individuals.82 Also, Ballaz et al.49 provided follow-up literature illustrating that long-term passive exercise interventions lead to significantly improved vascular function. This literature49, 81, 82, 83 strongly suggests that passive leg exercise increases femoral artery blood flow in people with SCI. Further research, with concurrent femoral artery flow measurements and isolated passive knee extension/flexion, is required to resolve this disagreement.
Finally, consideration must be given to the muscle groups stimulated while using FES. Three of the five papers used electrical stimulation sites at the hamstring, gluteal and quadriceps muscles,65, 71, 73 whereas De Groot et al.72 used tibialis anterior, gastrocnemius and quadriceps. Thijssen et al.75 have shown that when using FES, only the stimulated muscle tissue receives any improved arterial function or structure. Therefore, muscle groups used for electrical stimulation may be an important consideration when designing a rehabilitation program.
Conclusions
Cardiovascular disease, the primary cause of the death in those with SCI, is often overlooked in the SCI population owing to the multitude of other health issues and the focus on regenerating motor function. According to the available literature, exercise (upper body, electrically stimulated and passive modalities) improves arterial function in those with SCI. It cannot be overstated that more research should be performed investigating exercise as a means to improve arterial function in those with SCI as there is a clear shortage of published articles examining this relationship. Many exercise modalities have been explored by a single published article, and often studies lack matched or relevant control groups. Future investigations exploring exercise as a therapeutic rehabilitation technique for individuals with SCI should include the cost of, and accessibility to, equipment.
References
DeVivo MJ, Black KJ, Stover SL . Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 1993; 74: 248–254.
Nakanishi N, Kawashimo H, Nakamura K, Suzuki K, Yoshida H, Uzura S et al. Association of alcohol consumption with increase in aortic stiffness: a 9-year longitudinal study in middle-aged Japanese men. Ind Health 2001; 39: 24–28.
Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005; 43: 408–416.
Wilmer W, Nichols MFOR . Mcdonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Lea & Febiger: Philadelphia, PA, 2007.
Glasser SP, Arnett DK, McVeigh GE, Finkelstein SM, Bank AJ, Morgan DJ et al. Vascular compliance and cardiovascular disease: a risk factor or a marker? Am J Hypertens 1997; 10 (Part 1): 1175–1189.
Safar H, Mourad JJ, Safar M, Blacher J . Aortic pulse wave velocity, an independent marker of cardiovascular risk. Arch Mal Coeur Vaiss 2002; 95: 1215–1218.
Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993; 88 (Part 1): 1456–1462.
Ohmori K, Emura S, Takashima T . Risk factors of atherosclerosis and aortic pulse wave velocity. Angiology 2000; 51: 53–60.
Kesse-Guyot E, Vergnaud AC, Fezeu L, Zureik M, Blacher J, Peneau S et al. Associations between dietary patterns and arterial stiffness, carotid artery intima–media thickness and atherosclerosis. Eur J Cardiovasc Prev Rehabil 2010; 17: 718–724.
Tang W, Cheng LT, Lu XH, Wang T . Effect of nutrition on arterial stiffness in peritoneal dialysis patients. Am J Nephrol 2009; 30: 120–125.
Jatoi NA, Jerrard-Dunne P, Feely J, Mahmud A . Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007; 49: 981–985.
Yufu K, Takahashi N, Hara M, Saikawa T, Yoshimatsu H . Measurement of the brachial–ankle pulse wave velocity and flow-mediated dilatation in young, healthy smokers. Hypertens Res 2007; 30: 607–612.
Mahmud A, Feely J . Divergent effect of acute and chronic alcohol on arterial stiffness. Am J Hypertens 2002; 15: 240–243.
Mattace-Raso FU, van der Cammen TJ, van den Elzen AP, Schalekamp MA, Asmar R, Reneman RS et al. Moderate alcohol consumption is associated with reduced arterial stiffness in older adults: the Rotterdam study. J Gerontol A Biol Sci Med Sci 2005; 60: 1479–1483.
Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H et al. Central versus peripheral arterial stiffness in association with coronary, cerebral and peripheral arterial disease. Atherosclerosis 2010; 211: 480–485.
Gando Y, Yamamoto K, Murakami H, Ohmori Y, Kawakami R, Sanada K et al. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension 2010; 56: 540–546.
Theisen D, Vanlandewijck Y, Sturbois X, Francaux M . Blood distribution adaptations in paraplegics during posture changes: peripheral and central reflex responses. Eur J Appl Physiol 2000; 81: 463–469.
Gao SA, Ambring A, Lambert G, Karlsson AK . Autonomic control of the heart and renal vascular bed during autonomic dysreflexia in high spinal cord injury. Clin Auton Res 2002; 12: 457–464.
Jacobs PL, Nash MS . Exercise recommendations for individuals with spinal cord injury. Sports Med 2004; 34: 727–751.
Brown R, Macefield VG . Assessing the capacity of the sympathetic nervous system to respond to a cardiovascular challenge in human spinal cord injury. Spinal Cord 2008; 46: 666–672.
Kooijman M, Rongen GA, Smits P, Hopman MT . Preserved alpha-adrenergic tone in the leg vascular bed of spinal cord-injured individuals. Circulation 2003; 108: 2361–2367.
Wecht JM, Radulovic M, Lessey J, Spungen AM, Bauman WA . Common carotid and common femoral arterial dynamics during head-up tilt in persons with spinal cord injury. J Rehabil Res Dev 2004; 41: 89–94.
de Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT . Rapid and extensive arterial adaptations after spinal cord injury. Arch Phys Med Rehabil 2006; 87: 688–696.
Taylor PN, Ewins DJ, Fox B, Grundy D, Swain ID . Limb blood flow, cardiac output and quadriceps muscle bulk following spinal cord injury and the effect of training for the Odstock functional electrical stimulation standing system. Paraplegia 1993; 31: 303–310.
Rowell LB . Human Cardiovascular Control. Oxford University Press: London, 1993.
Pollack AA, Taylor BE, Myers TT, Wood EH . The effect of exercise and body position on the venous pressure at the ankle in patients having venous valvular defects. J Clin Invest 1949; 28: 559–563.
Folkow B, Gaskell P, Waaler BA . Blood flow through limb muscles during heavy rhythmic exercise. Acta Physiol Scand 1970; 80: 61–72.
Radegran G, Saltin B . Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol 1998; 274 (Part 2): H314–H322.
LaBarbera M . Principles of design of fluid transport systems in zoology. Science 1990; 249: 992–1000.
Martin TP, Stein RB, Hoeppner PH, Reid DC . Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol 1992; 72: 1401–1406.
Hopman MT, van Asten WN, Oeseburg B . Changes in blood flow in the common femoral artery related to inactivity and muscle atrophy in individuals with long-standing paraplegia. Adv Exp Med Biol 1996; 388: 379–383.
Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU . Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension 1997; 30: 1085–1088.
Lacolley P, Glaser E, Challande P, Boutouyrie P, Mignot JP, Duriez M et al. Structural changes and in situ aortic pressure–diameter relationship in long-term chemical-sympathectomized rats. Am J Physiol 1995; 269 (Part 2): H407–H416.
Aliev G, Ralevic V, Burnstock G . Depression of endothelial nitric oxide synthase but increased expression of endothelin-1 immunoreactivity in rat thoracic aortic endothelium associated with long-term, but not short-term, sympathectomy. Circ Res 1996; 79: 317–323.
Warburton DE, Nicol CW, Bredin SS . Health benefits of physical activity: the evidence. CMAJs 2006; 174: 801–809.
Warburton DER, Krassioukov A, Sproule S . Cardiovascular health and exercise rehabilitation in spinal cord injury. Top Spinal Cord Injury Rehabil 2007; 13: 98–122.
Gando Y, Kawano H, Yamamoto K, Sanada K, Tanimoto M, Oh T et al. Age and cardiorespiratory fitness are associated with arterial stiffening and left ventricular remodelling. J Hum Hypertens 2010; 24: 197–206.
Vivodtzev I, Minet C, Wuyam B, Borel JC, Vottero G, Monneret D et al. Significant improvement in arterial stiffness after endurance training in patients with COPD. Chest 2010; 137: 585–592.
Trzos E, Kurpesa M, Rechcinski T, Wierzbowska-Drabik K, Krzeminska-Pakula M . The influence of physical rehabilitation on arterial compliance in patients after myocardial infarction. Cardiol J 2007; 14: 366–371.
Kinzer SM, Convertino VA . Role of leg vasculature in the cardiovascular response to arm work in wheelchair-dependent populations. Clin Physiol 1989; 9: 525–533.
Hopman MT, Verheijen PH, Binkhorst RA . Volume changes in the legs of paraplegic subjects during arm exercise. J Appl Physiol 1993; 75: 2079–2083.
Moseley AM, Herbert RD, Sherrington C, Maher CG . Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother 2002; 48: 43–49.
Eng JJ, Teasell RJ, Miller WC, Wolfe DL, Townson AF, Aubut J et al. Spinal cord injury rehabilitation program: method of the SCIRE systematic review. Top Spinal Cord Injury 2007, 1–10.
Sackett DL, Rosenberg WMC, Gray JAM, Haynes BR, Richardson WS . Evidence based medicine: what it is and what it isn’t. BMJ 1996; 312: 71–72.
Krassioukov A, Warburton DE, Teasell R, Eng JJ . A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 2009; 90: 682–695.
Hing W, Bigelow R, Bremner T . Mulligan's mobilization with movement: a systematic review. J Man Manip Ther 2008; 17: E39–E66.
Hartling L, Brison RJ, Crumley ET, Klassen TP, Pickett W . A systematic review of interventions to prevent childhood farm injuries. Pediatrics 2004; 114: e483–e496.
Hignett S . Systematic review of patient handling activities starting in lying, sitting and standing positions. J Adv Nurs 2003; 41: 545–552.
Ballaz L, Fusco N, Cretual A, Langella B, Brissot R . Peripheral vascular changes after home-based passive leg cycle exercise training in people with paraplegia: a pilot Study. Arch Phys Med Rehabil 2008; 89: 2162–2166.
Foley NC, Teasell RW, Bhogal SK, Speechley MR . Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil 2003; 10: 1–7.
Bidart Y, Maury M . The circulatory behaviour in complete chronic paraplegia. II—circulatory adjustments to local metabolic needs. b—Muscular circulation. Paraplegia 1973a; 11: 1–24.
Bidart Y, Maury M . The circulatory behaviour in complete chronic paraplegia. IV—The loss of vasomotor adjustments to general needs. b—Study of arteriolar motricity. Paraplegia 1973b; 11: 1–24.
Ter Woerds WT, De Groot PCE, Van Kuppevelt DHJM, Hopman MTE . Passive leg movements and passive cycling do not alter arterial leg blood flow in subjects with spinal cord injury. Phys Ther 2006; 86: 636–645.
Dela F, Mohr T, Jensen CMR, Haahr HL, Secher NH, Biering-Sorensen F et al. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation 2003; 107: 2127–2133.
Nash MS, Montefusco CM, Rhodes BA . Peripheral vascular response to exercise-induced and postocclusive reactive hyperemia in quadriplegics. Surg Forum 1990; 41: 498–499.
Olive JL, Slade JM, Dudley GA, McCully KK . Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol 2003; 94: 701–708.
Ballaz L, Fusco N, Cretual A, Langella B, Brissot R . Acute peripheral blood flow response induced by passive leg cycle exercise in people with spinal cord Injury. Arch Phys Med Rehabil 2007; 88: 471–476.
Burkett LN, Chisum J, Pierce J, Pomeroy K, Fisher J, Martin M . Blood flow and lactic acid levels in exercising paralyzed wheelchair bound individuals. Adapt Phys Activ Q 1988; 5: 60–73.
Phillips W, Burkett LN, Munro R, Davis M, Pomeroy K . Relative changes in blood flow with functional electrical stimulation during exercise of the paralyzed lower limbs. Paraplegia 1995; 33: 90–93.
Svensson M, Siösteen H, Wetterqvist H, Sullivan L . Influence of physiotherapy on leg blood flow in patients with complete spinal cord injury lesions. Physiother Theory Pract 1995; 11: 97–107.
Seifert J, Lob G, Stoephasius E, Probst J, Brendel W . Blood flow in muscles of paraplegic patients under various conditions measured by a double isotope technique. Paraplegia 1972; 10: 185–191.
Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009; 19: 614–622.
Devillard X, Rimaud D, Roche F, Calmels P . Effects of training programs for spinal cord injury. Ann Readapt Med Phys 2007; 50: 490–498, 480–489.
Ditor DS, MacDonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005; 43: 664–673.
Zbogar D, Eng JJ, Krassioukov AV, Scott JM, Esch BTA, Warburton DER . The effects of functional electrical stimulation leg cycle ergometry training on arterial compliance in individuals with spinal cord injury. Spinal Cord 2008; 46: 722–726.
Nash MS, Jacobs PL, Montalvo BM, Klose KJ, Guest RS, Needham-Shropshire BM . Evaluation of a training program for persons with SCI paraplegia using the Parasteprho1 ambulation system: Part 5. Lower extremity blood flow and hyperemic responses to occlusion are augmented by ambulation training. Arch Phys Med Rehabil 1997; 78: 808–814.
Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK . Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord 2007; 45: 49–56.
Sabatier MJ, Stoner L, Mahoney ET, Black C, Elder C, Dudley GA et al. Electrically stimulated resistance training in SCI individuals increases muscle fatigue resistance but not femoral artery size or blood flow. Spinal Cord 2006; 44: 227–233.
Jae SY, Heffernan KS, Lee M, Fernhall B . Arterial structure and function in physically active persons with spinal cord injury. J Rehabil Med 2008; 40: 535–538.
Nash MS, Montalvo BM, Applegate B . Lower extremity blood flow and responses to occlusion ischemia differ in exercise-trained and sedentary tetraplegic persons. Arch Phys Med Rehabil 1996; 77: 1260–1265.
Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT . Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil 2001; 82: 832–839.
De Groot P, Crozier J, Rakobowchuk M, Hopman M, Macdonald M . Electrical stimulation alters FMD and arterial compliance in extremely inactive legs. Med Sci Sports Exerc 2005; 37: 1356–1364.
Hopman MT, Groothuis JT, Flendrie M, Gerrits KH, Houtman S . Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol 2002; 93: 1966–1972.
Thijssen DH, Ellenkamp R, Smits P, Hopman MT . Rapid vascular adaptations to training and detraining in persons with spinal cord injury. Arch Phys Med Rehabil 2006; 87: 474–481.
Thijssen DHJ, Heesterbeek P, Van Kuppevelt DJM, Duysens J, Hopman MTE . Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc 2005; 37: 1112–1118.
Tordi N, Mourot L, Chapuis A, Parratte B, Regnard J . Effects of a primary rehabilitation programme on arterial vascular adaptations in an individual with paraplegia. Ann Phys Rehabil Med 2009; 52: 66–73.
Krahn G, McCarthy M, Westwood D, Powers L . Evaluation of an innovative methodology to recruit research participants with spinal cord injury through durable medical equipment suppliers. Arch Phys Med Rehabil 2008; 89: 1341–1349.
Dearwater SR, LaPorte RE, Cauley JA, Brenes G . Assessment of physical activity in inactive populations. Med Sci Sports Exerc 1985; 17: 651–655.
Buchholz AC, McGillivray CF, Pencharz PB . Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res 2003; 11: 563–570.
Groothuis JT, Hopman MT . Does passive cycling induce changes in peripheral blood flow in persons with spinal cord injury? Arch Phys Med Rehabil 2007; 88: 1740.
Wong S, Krassioukov AV, Bredin SSD, Warburton DER . Temporal changes in cardiac output during arm cycle versus hybrid exercise in persons with spinal cord injury and able-bodied individuals. Med Sci Sports Exerc 2009; 41: s131.
Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R et al. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 2008; 586: 2405–2417.
Ballaz L . Does passive cycling induce changes in peripheral blood flow in persons with spinal cord injury? The author responds. Arch Phys Med Rehabil 2007; 88: 1741.
Acknowledgements
This research was supported by funding from the Canadian Institutes of Health Research, the Michael Smith Foundation for Health Research, the Natural Sciences and Engineering Research Council of Canada, the Canada Foundation for Innovation and the BC Knowledge Development Fund. DER Warburton was supported by salary awards from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. Special recognition is given to Drs Shannon Bredin and Sarah Charlesworth for their expertise with regard to the development of systematic reviews of the literature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Phillips, A., Cote, A. & Warburton, D. A systematic review of exercise as a therapeutic intervention to improve arterial function in persons living with spinal cord injury. Spinal Cord 49, 702–714 (2011). https://doi.org/10.1038/sc.2010.193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.193
Keywords
This article is cited by
-
Physical activity and cardiometabolic risk factors in individuals with spinal cord injury: a systematic review and meta-analysis
European Journal of Epidemiology (2022)
-
Vascular adaptations in nonstimulated areas during hybrid cycling or handcycling in people with a spinal cord injury: a pilot study of 10 cases
Spinal Cord Series and Cases (2021)
-
Acute effects of simultaneous electromyostimulation and vibration on leg blood flow in spinal cord injury
Spinal Cord (2016)
-
Peripheral vascular function in spinal cord injury: a systematic review
Spinal Cord (2013)