Abstract
Study design:
Prospective longitudinal experimental study.
Objectives:
The aim of this study was to assess the sensitivity to change of the electrical perceptual threshold (EPT) test during the longitudinal monitoring of neurological changes in patients with incomplete spinal cord injury (SCI).
Setting:
National Spinal Injuries Centre, Stoke Mandeville Hospital, Buckinghamshire Hospitals NHS Trust, Buckinghamshire, UK.
Methods:
Perceptual threshold to 3 Hz cutaneous electrical stimulation was measured in 11 patients with incomplete SCI at selected American Spinal Injuries Association (ASIA) sensory key points on four occasions. The first three measurements were performed within a 5-day period (baseline) and the fourth measurement (follow-up) at least 9 months later. The results were tested for statistical significance and the effect sizes were calculated.
Results:
There were no significant differences between the EPT results of the three baseline assessments. When the mean baseline and follow-up EPT results were compared, there were no significant differences in EPT values above the sensory level of lesion, but a significant difference (reduction in threshold values) was found at and below the level of SCI, with medium and large effect sizes, respectively.
Conclusion:
The EPT test showed good sensitivity to change in dermatomes at and directly below the sensory level of the SCI. This makes it a potentially useful quantitative sensory instrument for detecting changes in sensory function during longitudinal monitoring of patients with SCI.
Similar content being viewed by others
Introduction
The electrical perceptual threshold (EPT) test was adapted for use in patients with spinal cord injury (SCI) during the International Spinal Research Trust (ISRT) Clinical Initiative phase 1, as a quantitative sensory test that could complement the standard clinical sensory assessment in patients with SCI, particularly for research purposes.1, 2, 3
The need for improved and more sensitive outcome measures for SCI clinical trials has been emphasized repeatedly.4, 5, 6, 7 This is especially true of therapeutic clinical trials that, for safety reasons, may target patients with thoracic lesion, where current clinical assessment tools are not sensitive enough to detect minor neurological changes. The EPT test was developed with this in mind. So far, it has been shown to have good validity in assessing the sensory function following SCI2 and good intra- and inter-rater reliability in patients with SCI.3
The current study was a planned continuation of the reliability study.3 Its aim was to assess the sensitivity to change of the EPT test in a longitudinal study of subjects with incomplete SCI.
Materials and methods
The study was approved by the Mid and South Buckinghamshire Local Research Ethics Committee.
Subjects
Eleven of the twelve patient volunteers from the reliability study,3 all patients of the National Spinal Injuries Centre, Stoke Mandeville Hospital, were seen again for a follow-up EPT assessment. Only one patient from the previous reliability study, who had moved abroad, was not available to participate in the follow-up.
Experimental protocol
The study was designed as a prospective, longitudinal, test–retest study in order to measure the sensitivity to change (responsiveness) of the EPT over time. Each subject was tested on four separate occasions. The first three (baseline) assessments were part of the reliability study3 and for each participant the baseline tests were performed within a 5-day period and with an interval of at least 1 day between individual tests. The fourth (follow-up) assessment was performed between 9 and 25 months later, when the patients and the examiner were available for a follow-up appointment. Two of the three baseline assessments were performed by one examiner, and one baseline and the follow-up assessments, as well as the clinical neurological examination, by a second examiner.
Clinical assessment
The sensory level of injury for each side of the body and the American Spinal Injuries Association (ASIA) impairment scale (AIS) grade were determined at the time of the first baseline and the follow-up EPT assessment by the same examiner according to the International Standards for Neurological Classification of Spinal Cord injury (ISNCSCI).8
Electrical stimulation
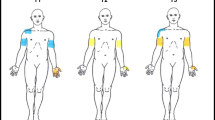
The technique used to determine the EPT was identical to the one used and described in detail in the reliability study.3 In short, square electrical pulses of 0.5 ms duration at 3 Hz frequency were applied to the skin via surface electrodes measuring 22 mm in diameter. The active electrode (cathode) was positioned over the ASIA sensory key points of selected dermatomes and the inactive anode remotely, on either forearm or lower leg, depending on which dermatomes were tested. The threshold was determined as the lowest electrical stimulus strength at which the subject reported a sensation of regular ‘light tapping or pulsating’ under the active electrode. In each subject, the EPT was measured in six dermatomes on both sides of the body. The dermatomes comprised of the dermatome identified at the baseline clinical examination as the sensory level of lesion, that is, the lowest spinal segment with normal sensation to both pin prick and light touch, and of the dermatomes immediately above and immediately below that level. All subjects also had the EPT measured in C4, T8 and L4 dermatomes, regardless of their level of injury.
The electrical stimulator used was a constant current stimulator (Model A395R, World Precision Instruments, Sarasota, FL, USA) controlled by a laptop computer to which it was connected via a data acquisition card (USB-6211, National Instruments, Austin, TX, USA). The software controlling the data acquisition card was written in-house and runs on the Windows XP Operating System (Microsoft, Richmond, VA, USA).
Using the method of limits, the subjects were asked to report immediately as soon as they first felt the sensation under the active electrode as the stimulus intensity was raised from 0 mA, and then again when the sensation disappeared as the stimulus intensity was lowered. The rates of ascent and descent were 0.1 mA per second and the preset upper stimulus intensity limit was 10 mA. On reporting the occurrence of sensation by the subject, the operator pressed the space bar on the laptop keyboard, which logged the output current of the stimulator. The computer software then continued to increase the output (3 times 0.1 mA) before reversing the change in intensity. When the subject reported a loss of sensation to the descending stimulus intensity, the output current was again logged. The procedure was repeated three times at each stimulated ASIA sensory point. The EPT used in further analysis was the mean of the three lowest stimulus intensities (in mA).
Statistical analysis
Descriptive statistics were used to present sample characteristics and the EPT results. Repeated-measures analysis of variance (ANOVA) was used to test statistically significant differences between the three-baseline EPT assessments. Paired sample t-test was used to test statistically significant differences between the mean baseline and the follow-up EPT results. The level of significance was set at P<0.05 and the Bonferroni correction was applied in primary analysis (Table 3) to adjust for multiple testing. The magnitude of change in EPT results was calculated using the effect size, d, defined as the mean of the paired differences divided by the standard deviation (s.d.) of the paired differences and with interpretation guidelines from Cohen:9 d of 0.2=small, 0.5=moderate and 0.8=large effect size.
Data were analysed using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
Sample characteristics
The basic demographic and SCI characteristics of the 11 patients with incomplete SCI seen for the follow-up assessment are presented in Table 1.
At baseline, the mean time since injury was 11.8 months (range 1–72) and the mean time from baseline to follow-up was 17.2 months (range 9–25). Between the baseline and the follow-up assessments, three patients, all with traumatic SCI, showed signs of clinical improvement in the sensory level of injury, having gained one to two levels, according to the clinical sensory examination. Two patients, one with traumatic and one with non-traumatic injury, showed clinical deterioration, having lost one to two sensory levels. The remaining six patients showed no change in sensory level of injury according to the clinical examination. No patients showed change in AIS grade between the baseline and follow-up assessments; two patients were classified as having AIS grade C, and nine patients as having AIS grade D.
EPT results
The baseline EPT results for the 11 patients who took part in the follow-up study were re-calculated from the results of the reliability study3 and are summarized in Table 2. There was no statistically significant difference between the three baseline EPT results (repeated-measures ANOVA, P>0.05).
As no significant difference was found between the three baseline EPT tests, the average (mean) value of the three tests was calculated and used for comparison with the follow-up EPT results (Table 3). Significant difference (reduction in threshold values) was found between the averaged baseline and the follow-up EPT results for dermatomes at and below the level of injury (P<0.05; paired sample t-test with Bonferroni correction), but not above the level of injury. The magnitude of the differences, as measured by the effect size, was small in the dermatomes above the level of injury, medium to large in the dermatomes at the level of injury and large in the dermatomes below the level of injury (Table 3).
Secondary analysis was performed on EPT results of the nine patients, who showed either improvement or no change clinically (after removing the data of the two patients who showed neurological deterioration), and the results were similar to those of the entire sample (Table 4).
Table 5 gives the mean baseline and follow-up EPT values for each of the three patient subgroups based on the clinical neurological change, but without statistical analysis, due to small patient numbers in each subgroup. Additionally, one patient who showed clinical deterioration lost previously recordable EPTs in dermatomes at and below the level of injury.
Figure 1 (scatter plot) shows the relationship between the change in EPT and the change in ASIA sensory grade for the dermatomes at, immediately above and below the sensory levels of injury. Both the deterioration and the improvement in clinical sensory results were usually accompanied by a change in EPT results in the same direction.
Scatter plot showing the relationship between the change in EPT (in mA) and change in ASIA sensory grade (0, 1 or 2) for each of the three analysed dermatomes (above, at and below the baseline level of injury). A negative change in EPT means an improvement (reduction in threshold values), while a negative change in ASIA sensory grade in clinical sensory testing means a deterioration.
Discussion
The aim of this study was to continue the development of the EPT test for use in patients with SCI by assessing its sensitivity to change (responsiveness) over time.
For an instrument to be useful as an outcome measure, it has to be valid, reliable and sensitive to change. The EPT test has a potential to be such an instrument. It has already proven construct validity2 and good intra- and inter-rater reliability in both control subjects1 and in patients with SCI.3 Leong et al.10 confirmed its good intra- and inter-rater reliability in control subjects and Kramer et al.11 showed it to have good predictive validity in patients with SCI. Recently, Kramer et al.12 studied dermatomal somatosensory evoked potentials (SEPs) and EPT during recovery from cervical SCI and concluded that the recordings of sensory innervation by dermatomal somatosensory evoked potentials and EPT provided reliable information about the preservation and changes in specific segments along the axis of the spinal cord.
This study provides further validation of the EPT technique by showing its sensitivity to change during the longitudinal follow-up of subjects with incomplete SCI. Additionally, it provides effect sizes, which are useful in carrying out a power calculation for future clinical trials of neurological recovery.
As already discussed in the reliability study,3 we specifically tested the dermatomes at, immediately above and immediately below the level of injury as the most relevant, given that these spinal cord segments were considered to be the ones most likely to show neurological change in the safety clinical trials anticipated by the ISRT.4 We did test in three fixed dermatomes (C4, T8 and L4) as well, targeting dermatomes well above and well below the injury level, but due to a mix of patients with very different levels of injury (between C1 and T12), we did not have enough cases for dermatomes C4 and T8 for statistical analysis, and in the dermatome L4 the pre-set upper stimulus intensity limit of 10 mA did not always yield a result. Therefore, the discussion concentrates mainly on the three dermatomes around the sensory level of injury.
The main study finding is that significant changes in EPT values over time, even when applying the Bonferroni correction, were found at and directly below the level of injury. Out of the 11 patients in our sample, 3 showed some clinical sensory improvement and gained back one to two segments, which is exactly what the improved EPT results at and immediately below injury level show. Most of the patients in this study did not show any clinically detectable improvement, but this does not necessarily mean that they did not have any. The validity study2 did suggest that EPT was able to detect small neurological deficits that are undetectable by clinical examination. In the same way, it may be able to record minor changes in sensory function that are undetectable by clinical examination. Both patients who showed clinical improvement and those who showed no change could have contributed to the lowering of EPT at and below the SCI level. Indeed, when the two patients who showed neurological deterioration were excluded from the analysis, the difference between the baseline and the follow-up assessment for the remaining nine patients was still statistically significant and the effect size of the improvement was even larger. Even the two patients whose clinical sensory level ascended showed some EPT improvement in dermatomes at and below the level of injury, but the statistical significance testing could not be performed, due to small patient numbers (Table 5, Figure 1).
At the same time, no statistically significant change in EPT and a small effect size was found in the dermatomes directly above the level of injury. A change in those dermatomes would be expected only if neurological deterioration occurred. Two patients in this study showed clinical deterioration and lost one to two segments of previously normal sensory function. When their EPT results were observed separately, they did show a very obvious rise in the mean EPT in the dermatomes above the level of injury (from 1.66 to 3.36 mA) between baseline and follow-up, but the numbers were too small for statistical analysis. This was in contrast to the patients who improved or showed no change clinically, both of whom showed slight improvement in EPT results above the level of injury, but which were not statistically significant (from 2.18 to 1.86 mA).
During this study, several measures were taken to minimize the confounding variability. Standardized stimulation sites were used, as in the two previous studies.2, 3 Additionally, a computer-controlled algorithm that further minimizes the rater variability was employed, as in the reliability study.3 The EPT test was already shown to have good intra- and inter-rater reliability in control subjects1, 10 and in patients with SCI.3, 12 In this study, no significant difference was found between the three baseline EPT results, which confirms its stability. Therefore, it is very likely that the longitudinal changes found in this study were due to genuine change in neurology. Also, the fact that significant differences between baseline and follow-up results were not found in dermatomes above the sensory level of injury, but only in those at and below the level, is a strong indication that the differences detected over time were most likely due to neurological changes. The only other published study that looked at longitudinal changes in EPT used a different methodology, but came to a similar conclusion that the EPT was responsive to the change in neurology over time, even though less so than the dermatomal SEPs that were also assessed in the same study.12
Study limitations
Sample size
The study design was such that it used the original sample of 12 patients from the reliability study3 and ended up with 11, due to unavailability of one patient for the follow-up. Larger patient numbers would allow a subgroup analysis of EPT sensitivity by neurological change (improvement/no change/deterioration), which was only partly possible here, as well as by level of injury. Still, even with a small study sample, statistically significant differences in EPT results were detected over time in dermatomes at and below, but not above, the SCI level.
Dermatomes tested
We specifically tested the dermatomes at, immediately above and immediately below the sensory level of injury for the reasons explained earlier in the Discussion section. In future studies, particularly those where neurological improvement and/or deterioration is expected, it would be informative to test for any changes in EPT results in dermatomes at least two or three segments below and above the level of injury, and calculate their effect size.
Upper stimulus intensity limit
We adhered to the original protocol from the two previous studies,2, 3 with the upper stimulus intensity limit of 10 mA, which could not always produce a response in the dermatome L4 and possibly some other more severely impaired dermatomes below the level of injury. Since then, it has been shown that the upper limit of 40 mA can be safely used, without any negative side effects.11, 12
Conclusions
The EPT test showed good sensitivity to change over time in dermatomes at and directly below the sensory level of SCI. This makes it a potentially useful quantitative sensory instrument for detecting changes in sensory function during the longitudinal monitoring of patients with SCI.
References
Davey NJ, Nowicky AV, Zaman R . Somatopy of perceptual threshold to cutaneous electrical stimulation in man. Exp Physiol 2001; 86: 127–130.
Savic G, Bergstrom EM, Frankel HL, Jamous MA, Ellaway PH, Davey NJ . Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord 2006; 44: 560–566.
King NK, Savic G, Frankel H, Jamous A, Ellaway P . Reliability of cutaneous electrical perceptual threshold in the assessment of sensory perception in patients with spinal cord injury. J Neurotrauma 2009; 26: 1061–1068.
Ellaway PH, Anand P, Bergstrom EMK, Catley M, Davey NJ, Frankel HL et al. Towards improved clinical and physiological assessments of recovery in spinal cord injury: a clinical initiative. Spinal Cord 2004; 42: 325–337.
Savic G, Bergström EMK, Davey NJ, Ellaway PH, Frankel HL, Jamous MA et al. Quantitative sensory tests (perceptual thresholds) in patients with spinal cord injury. J Rehabil Res Dev 2007; 44: 77–82.
Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221.
Alexander MS, Anderson KD, Biering-Sorensen F, Blight AR, Brannon R, Bryce TN et al. Outcome measures in spinal cord injury: recent assessments and recommendations for future directions. Spinal Cord 2009; 47: 582–591.
Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al., ASIA Neurological Standards Committee 2002. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26 (Suppl 1): S50–S56.
Cohen J . A power primer. Psychol Bull 1992; 112: 155–159.
Leong WS, Gorrie CA, Ng K, Rutkowski S, Waite PME . Electrical perceptual threshold testing: a validation study. J Spinal Cord Med 2009; 32: 140–146.
Kramer JLK, Moss AJ, Taylor P, Curt A . Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J Neurotrauma 2008; 25: 1019–1026.
Kramer JK, Taylor P, Steeves JD, Curt A . Dermatomal somatosensory evoked potentials and electrical perception thresholds during recovery from cervical spinal cord injury. Neurorehabil Neural Repair 2010; 24: 309–317.
Acknowledgements
We thank all the patient volunteers for taking part in the study. This work was supported by the Davenport Award from the Buckinghamshire Hospitals NHS Trust and by the Nathalie Rose Barr PhD Studentship from the ISRT, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Savic, G., Frankel, H., Jamous, M. et al. Sensitivity to change of the cutaneous electrical perceptual threshold test in longitudinal monitoring of spinal cord injury. Spinal Cord 49, 439–444 (2011). https://doi.org/10.1038/sc.2010.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.123
Keywords
This article is cited by
-
Discrepancies between clinical assessments of sensory function and electrical perceptual thresholds after incomplete chronic cervical spinal cord injury
Spinal Cord (2016)
-
Reliability of the electrical perceptual threshold and Semmes-Weinstein monofilament tests of cutaneous sensibility
Spinal Cord (2013)
-
The Sir Ludwig Guttmann Lecture 2012: the contribution of Stoke Mandeville Hospital to spinal cord injuries
Spinal Cord (2012)