Abstract
Study design:
Case report.
Objectives:
To report the late onset of cervical myelopathy secondary to fibrous scar tissue formation around an epidural electrode implanted for spinal cord stimulation (SCS).
Setting:
Department of Orthopaedic Surgery, Hoshigaoka Koseinenkin Hospital, Osaka, Japan.
Method and results:
A 49-year-old man who had an electrode implanted for SCS 5 years ago was referred to our department on 2 March 2005, complaining of difficulty using chopsticks and walking. A computed tomography scan with myelography revealed severe spinal cord compression around the epidural electrode. Surgical removal of the electrode was not effective. Removal of fibrous scar tissue during a second surgery significantly improved his neurological symptoms.
Conclusion:
Late onset cervical myelopathy secondary to fibrous scar tissue formation around the epidural electrode should be considered a possible event associated with SCS therapy.
Similar content being viewed by others
Introduction
Spinal cord stimulation (SCS) for the treatment of chronic pain has become an established modality since it was introduced in 1967. Although the incidence of adverse events associated with SCS therapy is high, serious neurological complications are rare.1, 2, 3, 4 We report the first case of cervical compression myelopathy at the site of the epidural electrode.
Case report
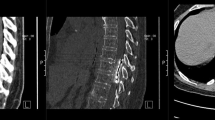
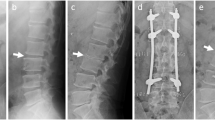
A 42-year-old man presented with a 7-year history of left arm pain secondary to a brachial plexus injury. The pain remained refractory to a comprehensive pain management plan. A computed tomography (CT) scan with myelography confirmed that there was no compression on the spinal cord (Figure 1a). On 21 September 2000, under general anesthesia, SCS was performed. An electrode (Medtrotonic Inc., Minneapolis, MN, USA) was positioned in the epidural space through the C4/5 interlamina space. Following a 7-day trial that showed SCS was beneficial in controlling his symptoms, the pulse generator was implanted. He then reported 80–90% pain relief. On 2 March 2005 (5 years after electrode implantation for SCS), he had difficulty using chopsticks and walking. Although a brain CT revealed no changes, he was referred to our department. Neurological examination revealed spastic quadriparesis with exaggerated deep tendon reflex in his lower extremities. CT scan with myelography revealed severe spinal cord compression from C1 to C5 by an epidural soft tissue mass (Figure 1b). We considered whether spontaneous resorption of the epidural mass after removal of the electrode might be possible. We discussed the option of decompression surgery or electrode-removal alone with the patient, and he chose the latter. His neurological symptoms were unchanged even after surgical removal of the electrode on 15 March 2005. Magnetic resonance imaging (MRI) revealed spinal cord compression (Figure 2a), and he was treated with cervical unilateral open-door laminoplasty from C3 to C6 on 16 May 2005. As severe adhesion between the dura and lamina was caused by scar tissue, we also removed it from the dura microscopically. He recovered gradually, and an MRI after surgery revealed the spinal cord was decompressed (Figure 2b). Histological examination of the epidural mass revealed fibrous scar tissue without inflammatory cells (Figure 3).
(a) Sagittal T2-weighted MRI scan before laminoplasty. The spinal cord is compressed from C1 to C5 by the epidural soft tissue mass. (b) Sagittal T2-weighted MRI scan after laminoplasty. The spinal cord is decompressed by the unilateral laminopasty from C3 to C6 and resection of the epidural soft tissue mass.
Discussion
The efficacy of SCS has been reported in the treatment of various chronic pain syndromes, including the neuropathic pain condition for which this patient was being treated.2 Although the incidence of adverse events associated with SCS therapy is high, the rate of serious complications is low.1, 2, 3, 4 North reported on the use of SCS for chronic, intractable pain and showed no major morbidity, such as spinal cord compression or injury, over 2 decades.1 The overall incidence of surgical wound infection was 5%, and the predominant complication has been electrode/lead assembly failure (7% by system) or radiofrequency receiver failure (5% by system). In a systemic review of SCS for failed back surgery syndrome or complex regional pain syndrome, Turner reported that 34% of patients (weighted average) who received a permanent stimulator had one or more complications during the follow-up period.3 The most common complication was a stimulator revision (23.1%) that required additional surgery. Although the rate of biological complications, such as dural puncture, was reported to be 5.8%, there were no reports of neurological complications. Similarly, Kumar4 reported a complication rate of 31.9% among 160 patients during a 10-year period. In his series, hardware-related complications accounted for 24.4% of problems, whereas biological complications accounted for 7.5%.4 There were no reports of neurological complications.4
Recently, Meyer5 published a case report about quadriparesis following revision of a spinal cord stimulator. This patient presented with upper and lower extremity weakness following inadvertent placement of an electrode into the spinal cord. The electrode was successfully removed; however, the neurological status deteriorated. Although Meyer5 first reported a neurological injury associated with SCS, the case seems to be the result of a complication related to a Touhy needle.
We report the first case of late onset cervical myelopathy secondary to fibrous scar tissue formation around the SCS electrode. In this case, we believe that the fibrous scar tissue was generated by an alien substance reaction to the electrode because there was no spinal cord compression on the myelogram before the electrode was implanted. Therefore, late onset cervical myelopathy secondary to fibrous scar tissue formation around the epidural electrode should be considered a possible event associated with SCS therapy.
Conclusion
Cervical myelopathy secondary to fibrous scar tissue formation around the epidural electrode is a possible event associated with SCS therapy. Physicians should be aware of late neurological deterioration in their surveillance of patients undergoing SCS therapy.
References
North RB, Kidd DH, Zahurak M, James CS, Long DM . Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery 1993; 32: 384–394; discussion 94-5.
ten Vaarwerk IA, Staal MJ . Spinal cord stimulation in chronic pain syndromes. Spinal Cord 1998; 36: 671–682.
Turner JA, Loeser JD, Deyo RA, Sanders SB . Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain 2004; 108: 137–147.
Kumar K, Wilson JR, Taylor RS, Gupta S . Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine 2006; 5: 191–203.
Meyer SC, Swartz K, Johnson JP . Quadriparesis and spinal cord stimulation: case report. Spine 2007; 32: E565–E568.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wada, E., Kawai, H. Late onset cervical myelopathy secondary to fibrous scar tissue formation around the spinal cord stimulation electrode. Spinal Cord 48, 646–648 (2010). https://doi.org/10.1038/sc.2009.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.188
Keywords
This article is cited by
-
Beyond treatment of chronic pain: a scoping review about epidural electrical spinal cord stimulation to restore sensorimotor and autonomic function after spinal cord injury
Neurological Research and Practice (2023)
-
Complications of Spinal Cord Stimulator Trials and Implants: A Review
Current Pain and Headache Reports (2023)
-
Complications of epidural spinal stimulation: lessons from the past and alternatives for the future
Spinal Cord (2020)