Abstract
Nanoparticles exhibit anisotropy when distinct features can be identified along different axes. Such disruption in shape and/or composition symmetry can change how nanoparticles behave and interact with the surrounding environment compared with their isotropic counterparts. Anisotropic combinations can be limitless and show potential for tackling biological barriers and developing programmable, targeted, and combined delivery of bioactive molecules, mainly when featuring autonomous motion. In this Review, we summarize the main methods for the generation of anisotropic particles at the nanoscale. We further discuss how geometric cues or the incorporation of propulsive agents (chemically or physically driven) improve transport across biological fluids, promote cellular adhesion and internalization, and/or increase tissue penetration. We finally highlight considerations for the design of anisotropic nanoparticles and the precise control over morphology and properties, in addition to the challenges for clinical translation.
Key points
-
Anisotropic systems exhibit specific spatial-dependent properties based on shape, chemical composition and/or physical responsiveness.
-
Precise engineering of anisotropic nanoparticles remains challenging and could benefit from the introduction of biocompatible materials.
-
Introducing a virtually unlimited combination array of anisotropic cues into nanoparticle design expands their applicability for combined drug delivery, targeting and theranostics.
-
Methodologies to assess nanoparticle–biological environment interactions and transport require further standardization for successful clinical translation.
Similar content being viewed by others
Introduction
Nanotechnology provides advanced tools and economic value to health-care systems1, where multiple products based on nanoparticles (NPs) as drug carriers have been approved for the treatment, prevention and diagnosis of diseases2. The most recent example comprises the COVID-19 vaccines from Pfizer/BioNTech, Moderna and Novavax.
NPs approved for medical use are mostly isotropic, with no variations in geometry or composition noted when observed from different angles. By contrast, anisotropy implies a directionality dependency3 (Box 1). In nature, anisotropy is dominant and assumed to result from adaptation to surrounding environmental cues. Anisotropy often provides advanced functionality; for instance, various bacteria, such as bacilli or spirochete, feature non-spherical shapes and/or specifically oriented surface structures, such as flagella or pili, that improve mobility in hard-to-tackle media4,5. The most recognizable anisotropic cells are erythrocytes, which have a biconcave disc shape that is linked to optimal flow in blood vessels6. Anisotropy also plays a role at the intracellular level to enhance transport. For example, biomolecular motors, such as myosin, dynein and kinesin, are responsible for the spatial organization of cellular organelles and vesicles, contributing to their directional and rapid displacement, particularly if clustered locally rather than randomly distributed7,8. Additionally, some viruses self-assemble into ordered prolate capsids that provide improved cargo protection and reduced free surface energy9. The highly pathogenic Ebola and Marburg viruses also rely heavily on their filamentous morphology to remain infective by featuring oriented cell entry and budding10,11.
The peculiar behaviour of anisotropic structures has inspired the development of highly functional materials. Anisotropic constructs hold unique characteristics in terms of shape, charge, hydrophilicity, and optical, electrical or magnetic properties, allowing applications involving micromotors12,13 and nanomotors14, tissue targeting15, responsive sensors16, stabilizers17 and other material building blocks18. Most anisotropic particles have dimensions in the micrometre range, facilitating the large-scale production of systems with improved control over shape and composition. However, such relatively large systems often do not meet size requirements for suitable biomedical applications, particularly for drug delivery, imaging, cell tagging and labelling, yet downsizing to the nanoscale poses substantial challenges in creating spatially distinct functionality, producing consistent size and shape, and yielding adequate response to stimuli19.
Nonetheless, interest in anisotropic nanomaterials for medical use is rising. For example, the non-spherical geometry of NPs can improve biodistribution and tune cell interactions20. Moreover, compositional anisotropy in spherical NPs can offer advantages over isotropic systems, including enabling tunable biodegradation and drug release21,22 or allowing specific bioactivity, such as the spatial presentation of ligands on pathogens owing to partial surface functionalization with targeting moieties23,24. Control over transport directionality of remotely guided or active colloids is also explored in this field25. Altogether, applications of anisotropic nanosystems include vaccine design26, production of platforms for cancer and photodynamic drug therapies27,28, and fighting antimicrobial resistance29.
Herein, we provide a critical overview of how anisotropic NPs can contribute to the design of advanced diagnostics and therapeutics and how these cues impact interactions with biological structures. We start by addressing the most relevant manufacturing techniques of anisotropic NPs (having at least a single dimension in the nanoscale range)30, which helps to better understand anisotropy and guides the scaling up of production processes. We then focus on how anisotropic NPs can tackle biological hurdles to perform their intended functions.
Production methods of anisotropic systems
Anisotropic NPs can be generated by various techniques that offer control over shape, composition or both (Fig. 1).
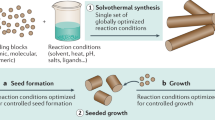
To produce shape anisotropy: a, Seed-mediated method; for example, gold stars35 and rods210 with improved optical properties are typically produced by seed-mediated methods. b, Lithography; for example, poly(lactic-co-glycolic acid) (PLGA) nanocylinders can be generated by nanoimprint lithography40. c, Self-assembly; for example, worms of controlled size can result from self-assembled spherical nanoparticles (NPs) made of thymine-containing polymers by adding adenine-containing polymer at specific adenine-to-thymine molar ratios48, and bowl-shaped stomatocytes result from shape transformation of self-assembled polymersomes, induced by osmotic shock109. d, Film stretching; for example, spherical polystyrene NPs can be elongated into discs by film-stretching methods59. To produce composition anisotropy: e, Template-assisted 2D method; for example, Janus-like mesoporous silica and iron oxide NPs can be partially coated with gold by depositing them in glass slides followed by toposelective sputtering60. f, Template-assisted 3D method; for example, Pickering emulsions are used to hide part of silica NPs74 for asymmetric surface functionalization generating Janus particles. g, Electric field application; for example, compartmental PLGA NPs80 featuring two distinct domains can be attained using electrohydrodynamic co-jetting. Part b adapted with permission from ref. 40, ACS. Part c (worm) adapted from ref. 48, Springer Nature Limited. Part g adapted with permission from ref. 80, Wiley.
Modulation of shape
Various shapes can be envisioned at the nanoscale. Non-spherical NPs can be obtained by bottom-up approaches such as the seeded growth method, where small seed particles act as nucleation sites that accumulate precursor molecules from a growth solution, growing into larger particles of controlled shape and size3. The technique can produce metallic stars and rods (Fig. 1a) with tunable surface plasmon resonance in the near-infrared (NIR) region. Owing to the ability of infrared light to penetrate tissue, the particles can be applied for cancer cell imaging31,32,33 and combinational photodynamic and photothermal therapy34,35.
Lithography-based techniques can generate NPs of precise shape and controlled dimensions by depositing a liquid precursor into a well-defined patterned mask, without the need for extreme temperatures and etching procedures. A common example is particle replication in non-wetting templates (PRINT), which enables scalable production of monodisperse trapezoidal and conical NPs of less than 100 nm in diameter. The precursor (for example, poly(ethylene glycol diacrylate), tri-acrylate resin, poly(lactic acid) or poly(pyrrole)) is cast onto a substrate and brought into contact with a patterned mould, generating NPs of the precursor material by mild heating, UV light crosslinking or solvent evaporation. Photocurable non-wetting and non-swelling moulds made of perfluoropolyether reduce the affinity to the liquid precursor, resulting in individualized particles36. PRINT is employed for the scalable production of polymeric NPs carrying proteins, such as avidin36 or small interfering RNA37, without affecting their bioactivity. For example, influenza vaccines based on poly(lactic-co-glycolic acid) (PLGA) and cholesterol cylindrical NPs (80 × 320 nm) have been developed by Liquidia Technologies38 using PRINT and tested in a phase I–II clinical trial (NCT01224262). Another interesting lithography technique is flash nanoimprinting, in which a release layer included in the substrate is dissolved at the end of the procedure, thus facilitating NP recovery from the moulds by avoiding the need for scraping. This technique is applied for the fabrication of stimuli-responsive, easily harvestable carriers of antibodies, nucleic acids39 and anticancer drugs40 (Fig. 1b). In combination with lithography techniques, template-assisted electrodeposition can be used to prepare elongated NPs or nanowires made of metals or semiconductor materials. In this technique, an electrolyte solution is reduced onto a conductive template substrate, growing according to its nanopattern. Finally, the template is dissolved or chemically etched41,42.
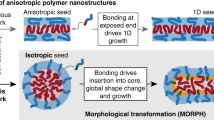
Anisotropic nanoconstructs, such as cylinders, worms, lamellae and rods, can also be generated spontaneously from amphiphilic block copolymers by self-assembly as promoted by energetic repulsion amongst different segments of copolymers, which leads to partitioning43,44. Autonomous spatial organization and the resulting shape depend on the monomers and the lengths of the polymer blocks. Other factors, such as temperature, pH, ionic strength or solvent, also play a role in the final features of constructs45,46. For instance, multicompartment micelles of ~100 nm in diameter can be prepared by hierarchical co-assembly of polycaprolactone-b-poly(ethylene glycol) (PCL-b-PEG) and PCL-b-poly(4-vinylpyridine). Shell micelles are first spontaneously formed owing to hydrophobic interactions among PCL chains, followed by pH adjustment to form phase-segregated patches. The multicompartment architecture of the obtained systems seems suitable for delivering incompatible compounds simultaneously47. Another example comprises the growth of anisotropic worms from spherical NPs made of amphiphilic block copolymers containing thymine by establishing supramolecular bonds with a second copolymer containing adenine at specific molar ratios (adenine-to-thymine ratio of between 0.2 and 1). The structure can be modified by incorporating bioactive or functional ligands for active drug delivery and targeting48 (Fig. 1c).
Alternative techniques, such as polymerization-induced self-assembly, are employed to generate drug-loaded NPs49. In this case, the polymeric block grows by adding monomers, becoming insoluble and leading to in situ assembly. For example, worm-like and rod-like micelles are prepared from poly(oligo(ethylene glycol)methacrylate)-b-(poly(styrene)-co-poly(vinyl benzaldehyde)) copolymers and further conjugated with doxorubicin50. Crystallization-driven self-assembly is also employed to prepare NPs of various morphologies (for example, diamond-like, platelet-like or cylindrical-shaped NPs) from poly(l-lactide)-based block copolymers, showing potential as platforms for drug delivery and immunotherapy owing to their shape-dependent macrophage activation51,52. Moreover, self-assembled anisotropic nanostructures can be made of shape memory polymers that can alter their conformation in response to specific stimuli. For example, jellyfish-like amphipathic NPs with diameters as small as 80 nm are prepared using a sucrose-PCL copolymer. The hydrophilic hydroxyl groups of sucrose protect the hydrophobic PCL tentacles in the centre, which recoil when abruptly exposed to negative pressures53. This strategy can be further adapted to generate NPs with shape memory that feature propulsive transport owing to dynamic shape-shifting upon external stimuli54.
Erythrocyte-shaped NPs (or stomatocytes) have been devised for active transport by entrapping metallic or biological catalysts that convert substrates into propulsive gases. Stomatocytes result from shape transformation of self-assembled spherical polymeric polymersomes that are susceptible to osmotic shock promoted by dialysis or electrolyte addition55 (Fig. 1c). These anisotropic particles have a bowl-like shape with a neck-like structure that shields the cargo against the surrounding environment55. Processing pre-prepared polymersomes through extrusion in a PEG solution triggers instantaneous osmotic shock and reduces the diameter to values below 200 nm, thus broadening their applicability56.
Film stretching is another approach to manipulate NP shape. In this technique, spherical polymeric particles are embedded in a continuous polymeric film and subjected to mechanical stress above their glass transition temperature or melting point such that they acquire the shape of the surrounding pocket holes after cooling57 (Fig. 1d). For example, various complex-shaped systems, such as prolate and oblate ellipsoidal NPs (inspired by erythrocytes), can be prepared by embedding spherical PLGA NPs into polyvinyl alcohol–glycerol films, followed by controlled stretching above the polyester melting point and cooling58. Despite its simplicity, one of the main drawbacks of the film-stretching technique concerns the complete removal of the materials used as embedding matrices, which might influence particle stability and size59.
Composition anisotropy
Focal changes to the surface composition of NPs can be achieved by topographically selective (toposelective) modification methods by which pre-prepared NPs are immobilized in 2D or 3D substrates for asymmetric functionalization. Such techniques rely on partial adhesion or embedment of NPs onto a template, allowing chemical modification of only the remaining exposed surface area. Deposition of NPs into glass or silicon slides followed by sputtering or electron-beam evaporation with gold is a common example60,61 (Fig. 1e). Other immobilization options could encompass particle embedment in poly-l-lysine–hyaluronic acid films for adsorption and/or functionalization of unmasked surfaces62,63, or attachment to UV-sensitive silicon nanosheets through hydrosilylation as employed for anisotropic functionalization of quantum dots64. Particles can then be released by ultrasonication, controlled desorption through sodium hydroxide exposure, or UV-induced template destruction.
Alternatively, polymeric microspheres can be used as 3D templates. For example, silica NPs down to 100 nm in diameter are partially labelled with gold NPs using polystyrene microsphere templates treated with supercritical carbon dioxide65. The glass transition temperature of polystyrene decreases upon exposure to carbon dioxide, hence allowing the control of NP embedment in the microsphere template by adjusting temperature and pressure. Subsequently, the partially exposed surface of the silica particles is modified with gold NPs. The same principle is employed to prepare strawberry-like structures, where iron oxide NPs, polystyrene and poly(acrylic acid) are used as coatings of silica NPs during functionalization66. Despite the popularity of polystyrene, polycarbonate microparticles are also described as templates67.
An alternative to polystyrene or polycarbonate microsphere templates is a fluid wax-in-water Pickering emulsion, a type of emulsion in which NPs act as stabilizers and accumulate at the oil–water interface to form colloidosomes. Paraffin is often chosen as the oil phase, although hexadecane68, dodecane69 and octanol17 can also be utilized. Stabilization of the dual phase system depends on the interfacial accumulation of NPs, and hardening of the inner oil phase can be further promoted by cooling, thus immobilizing the NPs70. The side of the NPs facing the aqueous phase of the emulsion can be functionalized, and anisotropic NPs can be collected by wax melting or solvent extraction, followed by filtration or centrifugation. In the case of magnetic NPs, the separation of the particles can be aided using a magnet. Silica is the most popular bulk material for NPs produced by such a technique but others of interest for drug delivery, such as zein71, PLGA72 and iron oxide73, are also considered. Pickering emulsion-based methods can produce large amounts of anisotropic NPs70. The technique can be applied to the production of pH-responsive NPs73 or systems with improved cell attachment and/or uptake74 (Fig. 1f).
Variations in composition can further extend to the bulk of NPs, particularly for compartmental systems. Electrospraying is used to produce dual compartmental spherical microparticles by applying a strong electric field to jetting liquids, leading to breakage of the stream into droplets75. These can be used to incorporate different molecules or supramolecular constructs of interest in opposing hemispheres of a polymeric core76,77,78. The production of such systems at the nanoscale, although challenging, is possible. Fine-tuning of the viscosity, conductivity and surface tension of jetting liquids as well as of the applied voltage and flow rates of electrospraying systems can result in particles of diameters as low as 100–200 nm without compromising the formation of individual compartments79. For example, the technique is applied to produce pH-responsive bicompartmental PLGA–acetal-modified dextran NPs (diameter of ~240 nm) for the co-delivery of anticancer drugs such as lapatinib and paclitaxel80 (Fig. 1g).
Combination systems
Systems of anisotropic shape and composition can be produced using a combination of different techniques. For example, dumbbell NPs are attained by selectively depositing cerium oxide or palladium at the tips of pre-prepared metallic nanorods, which spatially separates the structures involved in plasmonic photocatalysis and leads to a red-shift in the longitudinal localized surface plasmon resonance81,82. These systems have been proposed for photothermal therapies in the NIR region for Alzheimer disease and NIR photoacoustic imaging, respectively81,82. Similarly, Janus bimetallic nanorods made of gold and platinum have been prepared for deep tissue imaging exploiting the photoacoustic imaging capacity in the second NIR window, and chemotherapy induced by the cytotoxic effects of oxidized platinum (II) ions83. Hybrid metal–polymer NPs with a snowman-like configuration have also been produced, for example, by loading antibacterial silver cations into polymeric micelles followed by UV light exposure84, or by mixing platinum NPs with poly(divinylbenzene) to act as carriers of miconazole nitrate85, with both systems designed as antimicrobial therapies.
Seeded-emulsion polymerization techniques have also been used to prepare anisotropic NPs with distinctive compositions, for example, dumbbell-like and patchy particles for dual drug delivery86,87, and doxorubicin-loaded core-shell, snowman-like or dumbbell-like NPs (down to 400 nm) from 2-(dimethylamino)ethyl methacrylate and poly(2-hydroxyethyl methacrylate) seeds88. The technique is a two-step process in which seed particles are initially synthesized before swelling by a reactive monomer, resulting in the formation of bulges that are further polymerized89. Phase separation occurs owing to the incompatibility between seed particles and monomers and results in the formation of anisotropic NPs. The final shape is determined by interfacial tension forces so that the total free energy is minimized90. Another possible approach involves microfluidics fabrication. Despite being relatively inefficient in generating anisotropic particles at the nanoscale91,92, some successful attempts to produce nanosystems have been achieved93,94. Microfluidics may be specifically appealing when using polymers of distinct degradation rates and hydrophilicity for time-programmed dual drug delivery applications or to improve cargo release95,96. For example, ellipsoidal doxorubicin-loaded mesoporous silica NPs (average long axis of ~150 nm) with ordered parallel tunnels along their shorter axis have been prepared on spiral-shaped channels of a microfluidic reactor97.
Improving transport in fluids
The behaviour of NPs in biological fluids governs their ability to distribute and reach regions of interest, either at the site of administration or systemically. Transport of NPs in Newtonian biological fluids, such as urine or cerebrospinal fluid, depends largely on the viscosity of the medium rather than on the negligible inertial forces stemming from particulates. However, most biological fluids have non-Newtonian properties, such as blood, mucus and saliva, having rheological and mechanical features where time-reversible particle shape changes can generate propulsion98. As particle size decreases, environmental thermal fluctuations introduce variations to their movement, resulting in randomization of propulsion and uncertainty in transport characterization99. This can be partially addressed by introducing chemical modifications in particles (such as adding propulsive moieties to their composition) or media (for example, by generating gradients) to improve particle transport.
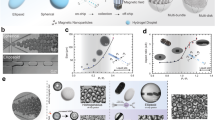
Particle engineering for transport modulation has led to the concept of (nano)motors, which can be defined as entities capable of transforming energy into motion99. A spherical particle experiences translational and rotational diffusion in liquid media, whose coefficients can be described by the Stokes–Einstein equation99. Such particles and the non-spherical ones can display improved transport beyond Brownian motion and be classified as motors, which is determined by their properties and/or propulsive forces. Nanomotor designs can be bioinspired. For example, biomolecular motors, such as kinesin, myosin, dynein and adenosine triphosphate synthase (ATPase), can provide efficient particle transport in aqueous environments through hydrolysis of ATP units100,101. These biosystems have inspired the design of synthetic nanomotors with propulsive and/or directional motion102,103 by converting external sources of energy into mechanical transport. Nanomotors are classified into two main classes: chemically fuelled (Fig. 2a), based on metal or enzymatic catalysis as a source of energy and inspired by microorganisms that harness chemical energy from the surrounding environment; and physically actuated (Fig. 2b), in which energy is provided by ultrasound, a magnetic field or light. Asymmetric reactivity resulting from geometric or surface anisotropy allows for directional transport owing to the imbalance in propulsive agent distribution across the particle. Nonetheless, this concept may be biased when considering nanosystems at a single particle level. The orientation of individualized NPs when introduced into biorelevant media is uncontrolled, leading to random directionality. Some strategies have been devised to tackle this issue, for example, by inducing chemotactic responses to local chemical gradients, which can be particularly relevant when targeting diseased areas that overexpress specific markers (for example, reactive oxygen species (ROS)104 or hydrogen peroxide105). Conferring magnetic properties to nanosystems is another approach that enables ensemble orientation or steering of nanomotors106,107 or, at least, can be used to minimize rotational diffusion of NPs108. Still, fluctuations in velocity and poor directional control of anisotropic NPs require further research, especially when considering transport in complex biological media109.
a, Chemically fuelled anisotropic systems. Improvement in the diffusive behaviour of nanoparticles (NPs) might stem from asymmetric chemical reactions occurring at the surface of particles, such as in the case of snowman-like (top left) or Janus-like motors (top right), or within a confined space such as in stomatocytes (bottom left and bottom right). Such reactions can be potentiated by inorganic or enzymatic catalysts. b, Physically actuated anisotropic systems. Remote control over NP dynamics can be achieved in physically actuated systems using, for example, ultrasound (left), a magnetic field (middle) or light (right). The arrows represent the direction of movement. All micrographs are from transmission electron microscopy, except for part b (left and middle) (scanning electron microscopy). Part a (top left) is adapted from ref. 112, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part a (bottom left) is adapted from ref. 117, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part a (top right) is adapted with permission from ref. 61, Elsevier. Part a (bottom right) is adapted from ref. 122, Springer Nature Limited. Part b (left) is adapted with permission from ref. 139, American Chemical Society. Part b (middle) is adapted with permission from ref. 142, American Chemical Society. Part b (right) is adapted with permission from ref. 154, Wiley.
Different approaches may help to improve the mobility of NPs for therapeutics and biomedical applications (Table 1). Irrespective of the strategy used to engineer anisotropic NPs with improved transport properties, a few drawbacks have limited applicability and further development of such nanosystems. These include the lack of precise control of propulsion in complex biological matrices, the need for dedicated equipment to generate power, and the use of toxic and/or scarcely available fuels.
Chemically fuelled anisotropic systems
Inorganically driven motors
NPs can be driven by chemophoretic mechanisms owing to catalytic reactions occurring in distinct regions populated with one or more propulsive agents. Inorganic materials, such as platinum and gold, can decompose hydrogen peroxide into molecular oxygen110,111,112 (Fig. 2a, top left), whereas magnesium, zinc or aluminium use water as fuel to generate molecular hydrogen113,114. For example, stomatocytes can be designed to entrap metallic catalysts that generate gaseous fluxes and thrust NPs unidirectionally for a limited period55 (Fig. 2a, bottom left).
Stomatocytes loaded with platinum NPs and functionalized with transactivating transcriptional activator cell-penetrating peptides have been tested for specific uptake by HeLa cancer cells115, which produce higher levels of hydrogen peroxide than non-cancerous cells. Similar stomatocytes loaded with doxorubicin showed higher directional mobility in the presence of hydrogen peroxide, with an estimated speed of up to 39 µm s–1 in aqueous media116. However, the inefficient encapsulation of platinum NPs and the harsh processing conditions, which may not be compatible with the incorporation of labile active molecules, could hamper the use of these stomatocytes. Alternatively, the inclusion of manganese dioxide NPs (25 nm in diameter) into PEG-b-poly(d,l-lactide)–polystyrene stomatocytes improves tumour penetration in HeLa cell-based spheroids. The inorganic NPs can generate oxygen by decomposing hydrogen peroxide, leading to variable autonomous motion related to fuel concentration, with particle velocities ranging from 15 to 20 µm s–1 for 5–50 mM of hydrogen peroxide117. In general, PEG is often included as particle shielding increases blood circulation time, controls drug release and lowers adhesive interactions with extracellular matrices118.
Enzyme-powered motors
Enzyme-based motors have a selective capability of substrate conversion. For example, catalase, urease and glucose oxidase are powerful propulsive units for thrusting NPs119, as seen for enzyme-fuelled hollow mesoporous silica NPs (<400 nm in diameter)120. These particles are half-capped with silicon dioxide and modified with either catalase, urease or glucose oxidase on the uncoated side. The NPs show an increasing diffusion coefficient (up to 1.30, 1.10 and 0.99 µm2 s–1, respectively) in aqueous solutions with increasing concentration of fuels (1.5 wt.% hydrogen peroxide, 25 mM urea or 500 mM glucose, respectively). Nonetheless, the motors require higher than physiological levels of urea and glucose to display noticeable propulsion. Still, urease-based and glucose oxidase-based NPs were biocompatible with HeLa cells120. In another study, streptavidin-coated magnetic NPs of 200 nm have been immobilized onto a magnet and asymmetrically coated with glucose oxidase. The remaining surface is functionalized with biotinylated peptides that target cell integrins. NPs show an increase of roughly 23% in the apparent diffusion coefficient with glucose concentration rising from 0 to 60 mM, which reduces binding between NPs and Chinese hamster ovary (CHO) cells121. Urease is a promising candidate to power particle motion. For example, a system composed of silica NPs sputter-coated with gold and functionalized with hyaluronic acid (for tumour targeting) on one side and urease on the other was developed for the delivery of camptothecin61 (Fig. 2a, top right). These Janus NPs feature an increase of 42% in diffusion coefficient (from 4.7 to 6.2 µm2 s–1) in phosphate-buffered saline upon increasing urea concentration from 5 to 10 mM (ref. 61).
A combination of enzymes can partially self-generate substrates that feed nanomotor activity122 (Fig. 2a, bottom right). For example, catalase and glucose oxidase have been encapsulated in nanosized stomatocytes109, where catalase breaks down hydrogen peroxide into water and oxygen, and glucose oxidase acts on glucose to generate gluconic acid and hydrogen peroxide. Thus, hydrogen peroxide can fuel the catalase reaction, making glucose the limiting fuel of the system. The nanomotor achieves a velocity of 6 µm s–1 with a glucose concentration as low as 5 mM without the need for exogenous hydrogen peroxide109.
Additional enzymes described as motors, including lipase123, histamine-metabolizing enzyme124 and cholesterol oxidase125, may also be promising for designing anisotropic nanosystems. Interestingly, above a certain threshold of enzyme concentration, these biocatalysts distribute at the surface of spherical particles in an unbalanced fashion, which is sufficient to cause the symmetry breakage necessary for active transport126. For example, urease-powered spherical nanosystems (that would otherwise be classified as isotropic) have been employed for intracellular drug delivery127, antibacterial activity128 and treating bladder cancer given the natural presence of urea129. Of note, an important aspect is the use of high-purity enzymes to achieve enhanced propulsive behaviour130.
Combining biological and inorganic catalysts can improve particle motion in fluids. For example, sub-micrometre systems have been developed by associating platinum NPs, glucose oxidase and a peptide-fuelled trypsin motor arranged in a Janus-like configuration. Further incorporation of manganese ferrite NPs enables control over directionality under the influence of a magnetic field by limiting particle rotational diffusion. Transport beyond Brownian motion is observed in the presence of 400 mM of glucose and 100 µM of (benzyloxycarbonyl-Ile-Pro-Arg)2-R110 (model peptide fuel)108, which are above physiological levels, highlighting the problem of most enzyme-based motors. Ideally, feasible nanomotors should be powered by readily available energy sources at biologically relevant levels131,132. Moreover, products stemming from enzymatic degradation, such as hydrogen peroxide or ammonia, may elicit toxicity133. Alternative fuels based on enzymatic reactions with biocompatible substrates are being explored and involve the use of external synthetic amino acids (for example, N-methacryl-l-arginine and N-methacryloyl-l-cysteine) that are converted to nitric oxide in the presence of its synthase or ROS. In this case, generated nitric oxide is not only useful for the propulsion of NPs but also acts as a bioactive agent owing to its vasodilating properties, which can potentiate cardiac targeting and angiogenesis applications104,134,135.
Physically actuated anisotropic systems
Toxicity associated with chemically fuelled systems and the variable or scarce availability of natural substrates at sites of interest have substantiated the development of fuel-free nanomotors that are responsive to external physical stimuli136.
Ultrasound
Low-power ultrasound, typically in the single-digit megahertz frequency range, can improve movement of anisotropic NPs136. Acoustically powered gold–nickel–gold nanowires can move through saliva and serum when submitted to ultrasound (10 V, 2.51 MHz), reaching speeds of approximately 10–50 µm s–1 (ref. 137). Furthermore, doxorubicin-loaded porous gold nanowires of similar dimensions have been designed for NIR light-triggered drug release. Movement results from the asymmetric pressure gradient at the concave extremity of the wire formed upon exposure to ultrasound, and the inherent porosity of the wires allows eightfold higher doxorubicin loading capacity. The nanocarriers can be directed towards adherent HeLa cells under ultrasound stimulus, showing potential for targeted drug delivery138. In another example, gold nanowires have been wrapped with a rolling circle amplification DNA strand and used as carriers for small interfering RNA delivery (Fig. 2b, left). The system can disrupt the cell membrane and circulate intra-cellularly upon ultrasound exposure, leading to 70–94% mRNA silencing (compared with less than 15% under static conditions) upon 5 min of treatment at 6 V and 2.66 MHz using green fluorescent protein-expressing HEK293 and MCF7 cell lines. The acoustic effect on membrane permeability remains unclear but potential cell damage has been dismissed as time and ultrasound intensity are deemed low139.
Magnetic field
Magnetism has been widely explored to guide NPs to sites of interest (for example, tumours) but it can also be used as an energy source to trigger the propulsion of anisotropic NPs140. This responsiveness of the particles initiates from the presence of magnetic materials in their composition. For instance, nanowires composed of a gold head and a nickel tail joined together by a silver bridge display magnetically driven propulsion under an external rotating 5 G magnetic field, arising from mechanical deformation and causing system symmetry breaking, thus inducing movement. These systems show high mobility in urine and hyperosmotic fluids, with speed increasing from 1.5 to 6 µm s–1 with varying magnetic frequency within the 5–15 Hz range141.
Magnetically driven helical silica–nickel propellers of nanoscaled size (100 × 400 nm) feature better directionality and mobility in synovial fluid surrogates than in a glycerol–water mixture, with velocity values increasing from 0.36 to 1.48 µm s–1 at 80 Hz (ref. 142) (Fig. 2b, middle). The small size of helical nanomotors makes magnetic field use impractical when aiming for directed synchronous motion in Newtonian fluids143. Additionally, nanomotors tend to aggregate, which limits their stability, particularly in hyperosmotic media141. This issue can be addressed by, for example, the addition of zinc ferrite coatings to silica–iron nanomotors, which increases particle stability for up to 6 months without impairing performance144. Such coatings can even improve haemocompatibility145 and qualify the helical nanomotors as cytocompatible146.
Magnetically activated nanomotors have also been applied to solid tumours. For example, the bioaccumulation of zinc-doped iron oxide nanocubes (60 nm) modified with the tripeptide arginine-glycine-aspartic acid (RGD) following intratumoural injection to U87 subcutaneous xenograft model on BALB/c mice was improved by the application of a rotating magnetic field (5 Hz) compared with the absence of a magnetic field or under a lower field strength (1 Hz). The nanocubes self-organize into elongated swarms capable of synchronous rotation under the magnetic field, thus promoting in situ residence and tumour growth suppression at 14 days post-injection147. However, the inefficiency of energy transfer across biological samples in deeper regions of the body (contrasting with the superficial tumour of the considered animal model) may impair magnetic activation147.
Light exposure
Light can power the motion of Janus particles featuring a metal-coated hemisphere owing to an asymmetric optothermal response that generates a propelling temperature gradient148,149,150. NIR light is typically selected owing to high tissue penetration depth and reduced photodamage, although UV radiation can also be useful for superficial applications151. Light-powered systems have mostly been developed at the microscale149 but nanomotors have also been explored. Nanoconstructs partially sputter-coated with gold have been designed for thermal ablation of cancer cells and photothermal therapy152,153. Janus gadolinium-doped, gold-coated mesoporous silica NPs have been developed for magnetic resonance imaging (Fig. 2b, right). The system shows solid tumour penetration by thermomechanical perforation of cell membranes when NPs are intravenously administered to BALB/c mice bearing subcutaneous 4T1 cell-derived tumours, and the diseased area is irradiated with NIR light154. Examples of other light-induced nanomotors include FoF1-ATPases asymmetrically distributed across the surface of polyelectrolyte-coated silica particles. Once exposed to NIR light, a generated proton gradient moves through the FoF1-ATPase channels, inducing their rotation and producing ATP. However, this approach has only been demonstrated for large particles (average diameter of 3 µm)155. Alternative strategies involve the development of hybrid nanomotors combining light with ultrasound156, a magnetic field157 or catalytic activation158. Combined physical stimuli offer control over particle aggregation and separation or the possibility of shifting between energy sources for better control of directionality158.
Tackling biological barriers
The fate of a nanosystem in the body depends on established interactions with the surrounding environment. Different anisotropic nanosystems have been shown to be effective for transport in biological fluids, cell internalization, overall biodistribution and immune evasion.
Transport across biological fluids
Different natural fluids can hinder the mobility of molecules and supramolecular structures, and various strategies have been devised to address such effects. Shape anisotropy can be beneficial for promoting permeation across mucus. For example, mesoporous silica nanorods (80 × 240 nm) show higher diffusivity and distribution in rat intestinal mucus than their 200-nm spherical counterparts composed of the same material and with similar surface charge and composition (Fig. 3a). Such behaviour is attributed to the rotational diffusion of nanorods promoted by shear flows and structural cues of the mucin network159. Similar results were observed for rod-shaped lovastatin nanocrystals with a hydrodynamic diameter of 400 nm. The nanostructures are produced by sonoprecipitation combined with high-pressure homogenization. Improved transport in rat mucus as well as cell and tissue uptake led to a higher oral bioavailability for the rod-shaped formulation160. Short self-assembled amphiphilic α-lactalbumin nanotubes (length of 100–200 nm) also demonstrate higher permeability in rat intestinal mucus than nanospheres or longer nanotubes. The nanotubes reach epithelial cells more effectively based on ex vivo mucus penetration studies and permeability assays in co-cultured monolayers of Caco-2 enterocyte-type and mucus-producing (as induced by methotrexate exposure) HT29-MTX cells. They also reveal the highest cell uptake and drug bioavailability when used as carriers for curcumin in Sprague–Dawley rats161. Once again, rotational diffusion — alongside size control that allows nanotubes to fit the tight aqueous channels created by the mucin mesh-like structure of mucus162 — appears to play an important role in improving transport.
a, Rods can permeate fresh rat intestinal mucus more efficiently than spheres of similar chemical composition and zeta potential (–5 mV). Both ensemble-average mean squared displacement (MSD) over time and distribution of the logarithms of single particle effective diffusivity (Deff) at a time scale of 1 s indicate improved transport of nanorods in mucus. Ex vivo particle distribution studies in rat intestinal mucus (in green) confirm that rods (in red, lower panel) exhibit improved penetration159. b, Tripeptide arginine-glycine-aspartic acid bearing gold nanoparticles (NPs) of varying aspect ratio (AR) are incubated with human mesenchymal stem cells (hMSCs) to assess anisotropic ligand presentation at the nanoscale. NPs with higher AR values potentiate efficient attachment of nanopodia (pointed out by white arrows) and result in improved cell adhesion. Following incubation of NPs with stem cells in NOD/SCID mice, cell adhesion and spreading are more pronounced for the groups of higher AR (AR4 and AR7) than in groups with lower AR (AR1 and AR2). Higher AR also translates into higher YES-associated protein (YAP) nuclear translocation, which is a mechanosensitive transcriptional factor for the regulation of cell fate towards specific cell lineages such as osteoblasts. Here, F-actin and NPs are coloured in red and yellow, respectively175. SD, Sprague–Dawley. Part a is adapted with permission from ref. 159, American Chemical Society. Part b is adapted with permission from ref. 175, Wiley.
The use of nanomotors to improve transport in mucus is also promising. For example, cisplatin-loaded self-propelled hollow mesoporous copper sulfide NPs (diameter <180 nm) half-capped with titanium have been proposed for thermophoretic mobility upon exposure to NIR light. These structures are further coated with cell membrane extracts from Staphylococcus aureus. Bacterial protein A can bind IgG, which is overexpressed in colorectal cancer, thus conferring tumour-targeting properties. Moreover, NPs show increased diffusivity in mucus surrogates in vitro with increasing laser power. These nanomotors inhibit tumour growth in orthotopic tumour-bearing mice following oral administration and NIR laser exposure163.
Anisotropy may also influence the way nanosystems behave when in blood circulation. Self-assembled cylinder-shaped filomicelles (20–60 nm × 8 µm) display blood circulation of up to 1 week, which is 10 times longer than that for spheres of similar composition. Particles with a length greater than 2.5 µm show the maximum half-life values of 5.2 days following intravenous injection to Sprague–Dawley rats or C57 mice. This is attributed to the structural similarities between filomicelles and natural filoviruses, as well as to controlled biodegradation (decrease in length) over time, until clearance. Moreover, paclitaxel-loaded filomicelles achieved tumour size regression in a lung cancer murine model164. In another study, elongated oleic acid-coated iron oxide nano-assemblies with superparamagnetic properties loaded with vincristine exhibited prolonged blood circulation residence over isotropic spherical counterparts, with an area under the plasma concentration–time curve and mean residence time values 1.2-fold and 1.7-fold higher, respectively, and a decrease of nearly 50% in clearance. Extended blood circulation of the drug carrier following intravenous administration contributes to the lowering of leukocyte count and improved expression of anti-leukaemia markers in a NOD/SCID leukaemia mouse model165. Similarly, prolonged blood circulation is observed for nanorods of PEG-b-dendritic polylysine–camptothecin (<500 nm in length) in comparison to spherical counterparts, with a half-life time of 5.8 h and 1.6 h, respectively, following intravenous administration to BALB/c mice166.
Nanoparticle–cell interactions
Unlike composition anisotropy, the impact of size, shape and aspect ratio of non-spherical NPs on cell attachment and uptake have been explored, although often leading to conflicting results167. As the NP shape can affect blood circulation time, disc-shaped mesoporous silicon particles (400 × 1,000 nm) show higher endothelial cell adhesion to diseased vasculature under in vitro microvascular flow conditions than rods or spherical counterparts. Complementary computational analysis suggests that the area of adhesion decreases from discs to rods to spheres as opposed to the dislodging hydrodynamic forces15. Similarly, platelet-like nanoporous silicon particles (400 × 1,000 nm) show higher accumulation in the tumour vasculature of melanoma-bearing mice168. The effect of shape on cell uptake has also been explored under non-flow dynamics such as in mesenchymal stem cells or HeLa cancer cells. For example, quasi-ellipsoidal NPs are less prone to undergo cell uptake than spherical counterparts, where the effect is related to the increasing aspect ratio59. Interestingly, studying the uptake of various anisotropic NPs under static or dynamic conditions yields different outcomes. For example, larger cuboid-shaped (800 × 100 × 100 nm) and disc-shaped (325 nm diameter × 100 nm height) PEG-diacrylate NPs exhibit higher uptake by endothelial cells than smaller particles (400 × 100 × 100 nm or 220 nm diameter × 100 nm height, respectively) under physiological-like shear flow conditions, although the contrary is observed for static conditions169. Moreover, in the presence of red blood cells and shear flow, oblate-shaped and rod-shaped biotin-functionalized polystyrene particles align and adhere more effectively to avidin-coated vessel walls of a microfluidic chamber than equivalent spherical particles. However, this effect is only evident for sizes exceeding 500 nm (ref. 170). Molecular simulations also suggest that penetration across cell membrane models is more effective for rods (1 nm × 3 nm), followed by discs (3 nm × 1 nm) and spheres (3 nm) of similar surface chemistry (composed of either polar, non-polar, charged or non-charged beads)171. Regarding antibacterial effects, short cylindrical micelles (length ≤4 µm) are more efficient in rupturing bacterial membranes and killing bacteria, as shown by their lower minimum inhibition concentration values against S. aureus, than their longer (length >10 µm) or spherical (200 nm) counterparts172.
Geometric anisotropy plays a definitive role in cell targeting of nanosystems by optimizing the presentation of targeting moieties to specific receptors173. For example, rod-shaped polystyrene NPs (approximately 130 × 370 nm) coated with the anti-receptor tyrosine-protein kinase ERBB2 antibody trastuzumab demonstrate higher specific uptake and surface binding when using different ERBB2+ breast cancer cell lines than discs or nanospheres174. In another example, an increase in the aspect ratio of gold nanorods coated with the cell adhesion motif RGD leads to augmented cell spreading, alignment of the basal cytoskeletal structure and maturation of cell focal adhesions owing to higher recruitment of β1 and β3 integrins when compared with spherical NPs (Fig. 3b). This also translates into higher expression of the osteogenic transcription factors Runt-related transcription factor 2 (RUNX2) and YES-associated protein, which promote stem cell differentiation into osteogenic lineage, showing the capacity of particle shape for tuning stem cell commitment175. In an inflammation cell culture model under dynamic conditions, polystyrene nanorods coated with antibodies to vascular adhesion molecule 1 (VCAM1), an endothelial inflammation marker, exhibit increased cell binding compared with their spherical counterparts and non-specific IgG-coated rod-like NPs. However, apart from the lungs, where VCAM1 rods show higher accumulation, no differences in biodistribution across other tissues (brain, kidney, liver and spleen) are observed for the different particle shapes following their intravenous administration in induced systemic inflammation mice models. These observations were attributed to the small differences in aspect ratio between the tested shapes176.
Overall, the definitive processes involved in anisotropic NP uptake by different cell types are still poorly understood. However, the combined analysis of factors, including particle shape, size, rigidity and surface chemistry, and cell properties (for example, preferential pathway for particle uptake, membrane flexibility and presence of receptors) can explain differences in uptake dynamics177,178. Hence, general guidance seems premature at this point without further extensive research.
Tissue penetration
Extracellular matrices and cell clusters constitute challenging barriers and anisotropy can provide a promising approach for NPs to bypass them. However, the systematization of evidence is challenging owing to the scarce data available on the topic and the plethora of particle design possibilities that are introduced by anisotropy. Some examples, however, provide compelling evidence of the effects that anisotropy can have on the biodistribution of NPs and support the use of such nanosystems for targeted delivery. For example, spherical, rod-shaped and star-shaped gold NPs administered intravenously to mice show different organ accumulation patterns179. Rods present lower biodistribution to tissues (including the liver) and rapid clearance, whereas spherical NPs accumulate mostly in the lungs but none of these NPs passes the blood–brain barrier (BBB)179. The findings may be attributed to the variable protein corona formed during blood circulation for differently shaped gold NPs180. Conversely, asymmetric snowman-like self-assembled vesicles (50 nm in diameter) made of poly(ethylene oxide)-poly(butylene oxide) with either poly((2-methacryloyl)ethyl phosphorylcholine)-poly(2-(diisopropylamino)ethyl methacrylate) or poly(oligo(ethylene glycol)methyl methacrylate)-poly(2-(diisopropylamino)ethyl methacrylate) and encapsulating glucose oxidase (with or without catalase) overcome the BBB. The system responds to external glucose gradients such as the positive gradient observed from the blood to the vessel wall and into the nervous system, thus allowing directional transport from the blood towards the brain181. The encapsulated enzymes react with their substrates in a confined space and produce a flow of hydrogen peroxide and gaseous oxygen that is preferentially expelled through the small and permeable bulge formed by poly(ethylene oxide)-poly(butylene oxide) of the anisotropic vesicle, thus generating propulsion. Favourable transport across the BBB is shown using an in situ perfusion rat model181. Even though composition and size are the most critical factors for overcoming the BBB182, anisotropic shapes may further help to access the nervous system by improving presentation183. For example, nanorods associate less extensively with the endothelium of a 3D BBB model than spherical NPs, but this only results in mild improvement in transport across the BBB184,185.
Aside from systems featuring geometric anisotropy, Janus-like NPs can be used as promising multifunctional platforms for theranostic applications. For example, silica NPs coated with gold in one hemisphere and further functionalized with a probe to sense hydrogen peroxide (3-mercaptophenylboronic acid), a photosensitizer (5,10,15,20-tetrakis(4-aminophenyl)porphyrin) and catalase have been developed (Fig. 4a). The system is responsive to the increased concentrations of hydrogen peroxide observed in the tumour microenvironment. Catalase decomposes hydrogen peroxide into oxygen, which improves transport and feeds the photosensitizer to produce ROS. Moreover, upon exposure to NIR light, the gold coating heats and induces a photothermal effect, promoting self-thermophoretic mobility. Additionally, fluorescent and photoacoustic imaging are possible owing to the presence of the gold nanoshell and the strong signal from the photosensitizer, respectively. Altogether, these NPs show synergistic photothermal and photodynamic effects, reducing tumour weight and size following intratumoural injection in a subcutaneous 4T1 breast tumour mouse model153. The addition of targeting moieties, such as affibodies, is also an interesting approach to promoting specific particle accumulation and, thus, improving the visualization and treatment of tumours186.
a, Dual-powered mesoporous silica nanoparticles (NPs) devised for theranostics. Half of the particle is coated with gold and functionalized with Raman reporter 3-mercaptophenylboronic acid (3-MPBA), which allows for surface-enhanced Raman scattering sensing (by detecting hydrogen peroxide (H2O2), used as a tumour marker) and enables photoacoustic imaging and photothermal therapy upon exposure to near-infrared (NIR) light. Irradiation also leads to excitation of the pre-loaded photosensitizer 5,10,15,20-tetrakis (4-aminophenyl) porphyrin (TAPP), thus generating reactive oxygen species. The addition of catalase to act as a photodynamic therapy (PDT) enhancer promotes the diffusion and deeper tumour penetration of the nanomotor through the release of oxygen (O2). The nanomotor is responsive in terms of diffusivity to both increasing concentrations of H2O2 and NIR light. Once injected intratumourally in 4T1 tumour-bearing mice, the full combinational therapy is the most promising regarding tumour volume and weight reductions compared with the use of NIR alone, combined with NPs without TAPP/catalase, or the full system without NIR153. b, Magnetic nanobullets are used for combined PDT and magnetic hyperthermia in an anti-metastatic immunotherapy approach. The mesoporous organosilica framework grows asymmetrically on iron oxide NPs and carries the photosensitizer chlorine e6. These structures are further cloaked with cancer cell membranes for homologous tumour-targeted accumulation and immune escape. Once exposed to a glutathione solution, which mimics the acidic and reductive tumour environment, nanobullets degrade and enable the release of chlorine e6. In vivo studies using an orthotropic 4T1 BALB/c mouse tumour model show that intravenous administration of the nanobullets and irradiation with NIR light in combination with an alternating current magnetic field (ACMF) and intravenous anti-CTLA4 antibody (an immune-checkpoint inhibitor) induce anticancer activity, reduce metastases in lungs and result in a sequence of immunogenic cell death25. MSD, mean squared displacement. Part a is adapted with permission from ref. 153, AAAS. Part b is adapted from ref. 25, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Anisotropic nanomotors can help penetration into pathological tissues and other tissue-like structures that are difficult to access. For example, peptide-modified polydopamine nano-bowls loaded with the nitric oxide donor N,N′-di-sec-butyl-N,N′-dinitroso-1,4-phenylenediamine have been developed for the treatment of thrombus. These nanomotors show propulsive properties upon NIR light stimulus as polydopamine absorbs and transfers energy into the nitric oxide donor and leads to the release of nitric oxide bubbles, resulting in deep thrombus infiltration and improved site-specific mechanical thrombolysis. The therapeutic effect in a tail thrombus mouse model outperformed clinical standard of care with recombinant tissue plasminogen activator, whilst avoiding recurrence owing to the anti-platelet adhesion and vasodilation effects of nitric oxide187. Considering the wound-healing properties of nitric oxide, calcium nanomotors have been partially capped with polydopamine grafted with cysteine-nitric oxide for S. aureus biofilm infiltration in diabetic foot ulcers of mice by decomposing calcium dioxide into molecular oxygen. The subsequent release of low levels of nitric oxide from polydopamine-g-cysteine-nitric oxide is also triggered by glutathione, which is naturally present in biofilms. Combined deep biofilm penetration of nanomotors and the intrinsic antibacterial activity of nitric oxide generated in situ results in accelerated wound closure188.
Immune response
The immune system can be seen as a scattered barrier to the biodistribution of particles, in which shape, size and their interrelation play a dominant role in determining immune cell uptake and influencing immunological outcomes189,190. For example, spheres and short filomicelles (diameter of ~60 nm and length of up to 2 μm) are more internalized by activated human-derived macrophages in vitro than long filomicelles (up to 3 μm) under flow conditions164. Similarly, the effect of aspect ratio on the interactions of stealth (PEG-coated) and targeted (RGD-coated) anisotropic nanorods with immune cells has been investigated in a colon cancer mouse model. The nanorods are produced by biotemplating through RNA-guided self-assembly of tobacco mosaic virus coat protein. The accumulation and penetration of PEG-coated nanorods in tumours is dependent on aspect ratio, with lower values outperforming higher ones in terms of transport from the blood vessel to the extravascular space. Tested aspect ratio values are 3.5, 7 and 16.5, corresponding to lengths varying between 60, 130 and 300 nm. Conversely, similar RGD-coated nanorods are quickly recognized by the immune system and eliminated. However, mid-sized (130 nm) RGD-coated rods feature the best size and ligand density combination, resulting in the highest specific tumour co-localization191.
Anisotropy not only influences immune cell uptake but can also trigger different immune responses. For example, 1D cylindrical glyco-NPs induce in vitro macrophage-mediated inflammation more pronouncedly than spherical counterparts despite being less internalized192. Moreover, 2D diamond-shaped glyco-platelets are poorly taken up by macrophages but lead to improved release of various pro-inflammatory cytokines (even more than for cylinder-shaped NPs), showing their potential for immune therapies51. These observations suggest that immune triggering may be induced by interactions at the cell membrane level, which are potentiated by the higher surface area-to-volume ratio of non-spherical constructs, without the need for uptake and intracellular processing. Similarly, ellipsoidal PLGA nanoconstructs produced by film stretching and used as antigen-presenting cell mimetics improve macrophage escape and antigen-specific induction of cytotoxic T lymphocytes. A strong immune cell response is further observed in irradiated THY1.2+ C57Bl/6 mice as an adoptive immunotherapy animal model after intravenous administration, contrasting with a mild response to spherical NPs193.
Overall, the distinct ability of anisotropic NPs to interact with immune cells opens new possibilities for immune engineering, with emphasis on the recent development of vaccines for cancer immunotherapy and infectious disease prevention. For example, antigen-presenting disc-shaped NPs made of apolipoprotein A1-mimetic peptide and phospholipids (diameter of ~10 nm) and co-loaded with aldehyde dehydrogenase (ALDH) antigen peptides and adjuvant molecules induce specific T cell responses and reduce cancer stem cells with a high expression of ALDH. The particles show antitumour efficacy in both D5 melanoma and 4T1 breast cancer murine models, particularly in combination with immune-checkpoint blockade with anti-PD-L1 (ref. 194). Similar systems incorporating human papillomavirus antigen improve the treatment of TC-1 mucosal tumours in C57Bl/6 mice as compared with the use of the soluble form of the antigen. Additionally, subcutaneous administration generates superior T cell responses compared with the same vaccine used by the intranasal route, which is only effective for the treatment of tumours that are in the vicinity of the vaccination site (namely for lung metastasis and sublingual inner lip tumours but not for intravaginal tumours)195. Treatment of gliomas has also been discussed by studies combining synthetic high-density lipoprotein nanodisc vaccines and chemotherapeutic agents196, or immune-checkpoint blockade with anti-PD-L1, particularly for personalized medicine approaches using endogenous antigens197. For infection prevention, vaccines based on gold nanorods have been designed as platforms to improve the efficacy of a synthetic RNA adjuvant following intranasal administration in mice. These vaccines can elicit stronger mucosal immune response against influenza virus infection as compared with similar spherical systems, even at lower antigen doses198.
Geometric anisotropic NPs can also be combined with magnetic responsiveness to induce hyperthermia in immunotherapy applications. An example is silica-based magnetic nanobullets carrying a photosensitizer intended for photodynamic therapy (Fig. 4b). After intravenous administration to 4T1 tumour-bearing mice, this system induces immunogenic cell death and leads to the elimination of deep metastatic tumours upon laser irradiation and application of a magnetic field in combination with immune-checkpoint inhibitors such as antibodies to CTLA4 (ref. 25).
Outlook
Introducing the concept of anisotropy into the engineering of nanomaterials opens a wide range of design possibilities for various biomedical applications. From creating smart multi-drug delivery systems for targeted therapies to developing tools for diagnostics, anisotropy allows modulation of the interactions of NPs with the surrounding environment and influences transport and distribution in biological settings. Once administered to complex organisms, engineered anisotropic nanosystems can reach target organs, tissues or even cell types, thus maximizing the chances for successful medical interventions. Overcoming well-known barriers, particularly by using nanomotors sensitive to remote guiding signals or featuring self-propulsion, represents a main direction of research in the field. Although external power sources offer some advantages (for example, real-time guidance control), the use of naturally occurring biomolecules to boost the movement of NPs seems the most promising approach for achieving active and directional transport. However, evidence for this derives from studies performed using surrogate media that fall short in representing complex biological environments142. Improving transport and control over the directionality of nanomotors is often close to the limits of experimental reproducibility and statistical significance, making it hard to distinguish between experimental bias and actual highly diffusive and oriented phenomena99. Furthermore, NP fabrication typically involves multistep and complex processes, which leads to low yields and poor reproducibility. Thus, these and other issues should be considered to allow the translation of anisotropic NPs into the clinic (Box 2). Overall, the introduction of anisotropic principles in the design process of NPs holds potential to endow nanomedicines with a whole new set of functionalities that may contribute to improved therapeutics, prophylaxis and diagnosis.
References
Talebian, S. et al. Facts and figures on materials science and nanotechnology progress and investment. ACS Nano 15, 15940–15952 (2021).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, e10143 (2019).
Pearce, A. K., Wilks, T. R., Arno, M. C. & O’Reilly, R. K. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nat. Rev. Chem. 5, 21–45 (2020).
Wadhwa, N. & Berg, H. C. Bacterial motility: machinery and mechanisms. Nat. Rev. Microbiol. 20, 161–173 (2022).
Constantino, M. A., Jabbarzadeh, M., Fu, H. C. & Bansil, R. Helical and rod-shaped bacteria swim in helical trajectories with little additional propulsion from helical shape. Sci. Adv. 2, e1601661 (2016).
Mesarec, L. et al. Normal red blood cells’ shape stabilized by membrane’s in-plane ordering. Sci. Rep. 9, 19742 (2019).
Iino, R., Kinbara, K. & Bryant, Z. Introduction: molecular motors. Chem. Rev. 120, 1–4 (2020).
Erickson, R. P., Jia, Z., Gross, S. P. & Yu, C. C. How molecular motors are arranged on a cargo is important for vesicular transport. PLoS Comput. Biol. 7, e1002032 (2011).
Luque, A., Zandi, R. & Reguera, D. Optimal architectures of elongated viruses. Proc. Natl Acad. Sci. USA 107, 5323–5328 (2010).
Welsch, S. et al. Electron tomography reveals the steps in filovirus budding. PLoS Pathog. 6, e1000875 (2010).
Zhang, Q. et al. Entry dynamics of single Ebola virus revealed by force tracing. ACS Nano 14, 7046–7054 (2020).
Choi, H., Jeong, S. H., Kim, T. Y., Yi, J. & Hahn, S. K. Bioinspired urease-powered micromotor as an active oral drug delivery carrier in stomach. Bioact. Mater. 9, 54–62 (2022).
Walker, D., Käsdorf, B. T., Jeong, H. H., Lieleg, O. & Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 1, e1500501 (2015).
Lin, R., Yu, W., Chen, X. & Gao, H. Self-propelled micro/nanomotors for tumor targeting delivery and therapy. Adv. Healthc. Mater. 10, e2001212 (2021).
Adriani, G. et al. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials 33, 5504–5513 (2012).
Thome, C. P., Hoertdoerfer, W. S., Bendorf, J. R., Lee, J. G. & Shields, C. W. Electrokinetic active particles for motion-based biomolecule detection. Nano Lett. 23, 2379–2387 (2023).
Ifra, Thodikayil, A. T. & Saha, S. Compositionally anisotropic colloidal surfactant decorated with dual metallic nanoparticles as a pickering emulsion stabilizer and their application in catalysis. ACS Appl. Mater. Interfaces 14, 23436–23451 (2022).
Glotzer, S. C. & Solomon, M. J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 6, 557–562 (2007).
Hu, S.-H. & Gao, X. Nanocomposites with spatially separated functionalities for combined imaging and magnetolytic therapy. J. Am. Chem. Soc. 132, 7234–7237 (2010).
Decuzzi, P. et al. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 141, 320–327 (2010).
Zhang, L. et al. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials 181, 113–125 (2018).
Winkler, J. S., Barai, M. & Tomassone, M. S. Dual drug-loaded biodegradable Janus particles for simultaneous co-delivery of hydrophobic and hydrophilic compounds. Exp. Biol. Med. 244, 1162–1177 (2019).
Jiao, M., Li, W., Yu, Y. & Yu, Y. Anisotropic presentation of ligands on cargos modulates degradative function of phagosomes. Biophys. Rep. 2, 100041 (2022).
Shaghaghi, B., Khoee, S. & Bonakdar, S. Preparation of multifunctional Janus nanoparticles on the basis of SPIONs as targeted drug delivery system. Int. J. Pharm. 559, 1–12 (2019).
Wang, Z. et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv. Sci. 6, 1901690 (2019).
Niikura, K. et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 7, 3926–3938 (2013).
Zhang, W., Choi, H., Yu, B. & Kim, D.-H. Synthesis of iron oxide nanocube patched Janus magnetic nanocarriers for cancer therapeutic applications. Chem. Comm. 56, 8810–8813 (2020).
Zhang, M. et al. Precise synthesis of unique polydopamine/mesoporous calcium phosphate hollow Janus nanoparticles for imaging-guided chemo-photothermal synergistic therapy. Chem. Sci. 8, 8067–8077 (2017).
Rossi, F., Khoo, E. H., Su, X. & Thanh, N. T. K. Study of the effect of anisotropic gold nanoparticles on plasmonic coupling with a photosensitizer for antimicrobial film. ACS Appl. Bio Mater. 3, 315–326 (2020).
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A. & Danquah, M. K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9, 1050–1074 (2018).
Harmsen, S. et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci. Transl Med. 7, 271ra277 (2015).
Tian, Y. et al. Gold nanostars functionalized with amine-terminated PEG for X-ray/CT imaging and photothermal therapy. J. Mater. Chem. B 3, 4330–4337 (2015).
Huang, X., El-Sayed, I. H., Qian, W. & El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128, 2115–2120 (2006).
Soleimany, A., Khoee, S., Dias, S. & Sarmento, B. Exploring low-power single-pulsed laser-triggered two-photon photodynamic/photothermal combination therapy using a gold nanostar/graphene quantum dot nanohybrid. ACS Appl. Mater. Interfaces 15, 20811–20821 (2023).
Li, Z. et al. Ce6-conjugated and polydopamine-coated gold nanostars with enhanced photoacoustic imaging and photothermal/photodynamic therapy to inhibit lung metastasis of breast cancer. Nanoscale 12, 22173–22184 (2020).
Rolland, J. P. et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 127, 10096–10100 (2005).
Hasan, W. et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 12, 287–292 (2012).
Galloway, A. L. et al. Development of a nanoparticle-based influenza vaccine using the PRINT technology. Nanomedicine 9, 523–531 (2013).
Glangchai, L. C., Caldorera-Moore, M., Shi, L. & Roy, K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J. Control. Release 125, 263–272 (2008).
Zhang, X. et al. Controllable subtractive nanoimprint lithography for precisely fabricating paclitaxel-loaded PLGA nanocylinders to enhance anticancer efficacy. ACS Appl. Mater. Interfaces 12, 14797–14805 (2020).
Esteban-Fernández de Ávila, B. et al. Nanomotor-enabled pH-responsive intracellular delivery of caspase-3: toward rapid cell apoptosis. ACS Nano 11, 5367–5374 (2017).
Ruiz-Gómez, S., Fernández-González, C. & Perez, L. Electrodeposition as a tool for nanostructuring magnetic materials. Micromachines 13, 1223 (2022).
Kang, C. & Honciuc, A. Self-assembly of Janus nanoparticles into transformable suprastructures. J. Phys. Chem. Lett. 9, 1415–1421 (2018).
Li, Z., Kesselman, E., Talmon, Y., Hillmyer, M. A. & Lodge, T. P. Multicompartment micelles from ABC miktoarm stars in water. Science 306, 98–101 (2004).
Khoee, S. & Nouri, A. in Design and Development of New Nanocarriers (ed. Grumezescu, A. M.) 145–180 (Elsevier, 2018).
Cui, H., Chen, Z., Zhong, S., Wooley, K. L. & Pochan, D. J. Block copolymer assembly via kinetic control. Science 317, 647–650 (2007).
Liu, X. et al. Multicompartment micelles based on hierarchical co-assembly of PCL-b-PEG and PCL-b-P4VP diblock copolymers. RSC Adv. 6, 5312–5319 (2016).
Hua, Z. et al. Anisotropic polymer nanoparticles with controlled dimensions from the morphological transformation of isotropic seeds. Nat. Commun. 10, 5406 (2019).
Penfold, N. J. W., Yeow, J., Boyer, C. & Armes, S. P. Emerging trends in polymerization-induced self-assembly. ACS Macro Lett. 8, 1029–1054 (2019).
Karagoz, B. et al. Polymerization-induced self-assembly (PISA) – control over the morphology of nanoparticles for drug delivery applications. Polym. Chem. 5, 350–355 (2014).
Li, Z. et al. Glyco-platelets with controlled morphologies via crystallization-driven self-assembly and their shape-dependent interplay with macrophages. ACS Macro Lett. 8, 596–602 (2019).
Inam, M. et al. 1D vs. 2D shape selectivity in the crystallization-driven self-assembly of polylactide block copolymers. Chem. Sci. 8, 4223–4230 (2017).
Zhang, J. et al. Shape memory actuation of Janus nanoparticles with amphipathic cross-linked network. ACS Macro Lett. 5, 1317–1321 (2016).
Yan, B. et al. Investigating switchable nanostructures in shape memory process for amphipathic Janus nanoparticles. ACS Appl. Mater. Interfaces 10, 36249–36258 (2018).
Keller, S., Toebes, B. J. & Wilson, D. A. Active, autonomous, and adaptive polymeric particles for biomedical applications. Biomacromolecules 20, 1135–1145 (2018).
Sun, J., Mathesh, M., Li, W. & Wilson, D. A. Enzyme-powered nanomotors with controlled size for biomedical applications. ACS Nano 13, 10191–10200 (2019).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl Acad. Sci. USA 104, 11901–11904 (2007).
Ben-Akiva, E. et al. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 6, eaay9035 (2020).
Florez, L. et al. How shape influences uptake: interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 8, 2222–2230 (2012).
Sun, Z. et al. Self-propelled Janus nanocatalytic robots guided by magnetic resonance imaging for enhanced tumor penetration and therapy. J. Am. Chem. Soc. 145, 11019–11032 (2023).
Chen, Z. et al. Enzyme-powered Janus nanomotors launched from intratumoral depots to address drug delivery barriers. Chem. Eng. J. 375, 122109 (2019).
Delcea, M. et al. Anisotropic multicompartment micro- and nano-capsules produced via embedding into biocompatible PLL/HA films. Chem. Comm. 47, 2098–2100 (2011).
Tang, S. et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot. 5, eaba6137 (2020).
Kloberg, M. J. et al. Surface-anisotropic Janus silicon quantum dots via masking on 2D silicon nanosheets. Adv. Mater. 33, e2100288 (2021).
Yang, Q., de Vries, M. H., Picchioni, F. & Loos, K. A novel method of preparing metallic Janus silica particles using supercritical carbon dioxide. Nanoscale 5, 10420–10427 (2013).
Yang, Q., Miao, X. & Loos, K. Fabrication of nano-sized hybrid Janus particles from strawberry-like hierarchical composites. Macromol. Chem. Phys. 219, 1800267 (2018).
Mani, K. A., Yaakov, N., Itzhaik Alkotzer, Y., Zelikman, E. & Mechrez, G. A robust fabrication method for amphiphilic Janus particles via immobilization on polycarbonate microspheres. Polymers 10, 900 (2018).
Kalashnikova, I., Bizot, H., Bertoncini, P., Cathala, B. & Capron, I. Cellulosic nanorods of various aspect ratios for oil in water pickering emulsions. Soft Matter 9, 952–959 (2013).
Hunter, S. J. & Armes, S. P. Pickering emulsifiers based on block copolymer nanoparticles prepared by polymerization-induced self-assembly. Langmuir 36, 15463–15484 (2020).
Jiang, S. & Granick, S. Controlling the geometry (Janus balance) of amphiphilic colloidal particles. Langmuir 24, 2438–2445 (2007).
Zhao, Z., Wang, W., Xiao, J., Chen, Y. & Cao, Y. Interfacial engineering of pickering emulsion co-stabilized by zein nanoparticles and Tween 20: effects of the particle size on the interfacial concentration of gallic acid and the oxidative stability. Nanomaterials 10, 1068 (2020).
Robin, B. et al. Tuning morphology of Pickering emulsions stabilised by biodegradable PLGA nanoparticles: how PLGA characteristics influence emulsion properties. J. Colloid Interface Sci. 595, 202–211 (2021).
Cai, S. et al. pH-responsive superstructures prepared via the assembly of Fe3O4 amphipathic Janus nanoparticles. Regen. Biomater. 5, 251–259 (2018).
Kadam, R., Ghawali, J., Waespy, M., Maas, M. & Rezwan, K. Janus nanoparticles designed for extended cell surface attachment. Nanoscale 12, 18938–18949 (2020).
Wang, J., Jansen, J. A. & Yang, F. Electrospraying: possibilities and challenges of engineering carriers for biomedical applications-a mini review. Front. Chem. 7, 258 (2019).
Sanchez-Vazquez, B., Amaral, A. J. R., Yu, D. G., Pasparakis, G. & Williams, G. R. Electrosprayed Janus particles for combined photo-chemotherapy. AAPS PharmSciTech 18, 1460–1468 (2017).
Li, K. et al. Enhanced fluorescent intensity of magnetic-fluorescent bifunctional PLGA microspheres based on Janus electrospraying for bioapplication. Sci. Rep. 8, 17117 (2018).
Hwang, S. et al. Anisotropic hybrid particles based on electrohydrodynamic co-jetting of nanoparticle suspensions. Phys. Chem. Chem. Phys. 12, 11894–11899 (2010).
Roh, K. H., Martin, D. C. & Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 4, 759–763 (2005).
Gregory, J. V. et al. Programmable delivery of synergistic cancer drug combinations using bicompartmental nanoparticles. Adv. Healthc. Mater. 9, e2000564 (2020).
Ge, K. et al. Gold nanorods with spatial separation of CeO2 deposition for plasmonic-enhanced antioxidant stress and photothermal therapy of Alzheimer’s disease. ACS Appl. Mater. Interfaces 14, 3662–3674 (2022).
Ye, J. et al. Quantitative photoacoustic diagnosis and precise treatment of inflammation in vivo using activatable theranostic nanoprobe. Adv. Funct. Mater. 30, 2001771 (2020).
Li, Q. et al. Nanosized Janus AuNR-Pt motor for enhancing NIR-II photoacoustic imaging of deep tumor and Pt2+ ion-based chemotherapy. ACS Nano 16, 7947–7960 (2022).
Li, R. et al. In situ production of Ag/polymer asymmetric nanoparticles via a powerful light-driven technique. J. Am. Chem. Soc. 141, 19542–19545 (2019).
Ji, X. et al. Multifunctional parachute-like nanomotors for enhanced skin penetration and synergistic antifungal therapy. ACS Nano 15, 14218–14228 (2021).
Dehghani, E., Salami-Kalajahi, M. & Roghani-Mamaqani, H. Simultaneous two drugs release form Janus particles prepared via polymerization-induced phase separation approach. Colloids Surf. B 170, 85–91 (2018).
Dehghani, E., Salami-Kalajahi, M. & Roghani-Mamaqani, H. Fabricating cauliflower-like and dumbbell-like Janus particles: loading and simultaneous release of DOX and ibuprofen. Colloids Surf. B 173, 155–163 (2019).
Dehghani, E., Barzgari-Mazgar, T., Salami-Kalajahi, M. & Kahaie-Khosrowshahi, A. A pH-controlled approach to fabricate electrolyte/non-electrolyte Janus particles with low cytotoxicity as carriers of DOX. Mater. Chem. Phys 249, 123000 (2020).
Li, Y. et al. Morphology evolution of Janus dumbbell nanoparticles in seeded emulsion polymerization. J. Colloid Interface Sci. 543, 34–42 (2019).
Vatankhah, Z., Dehghani, E., Salami-Kalajahi, M. & Roghani-Mamaqani, H. Seed’s morphology-induced core-shell composite particles by seeded emulsion polymerization for drug delivery. Colloids Surf. B 191, 111008 (2020).
Meyer, R. A. & Green, J. J. Shaping the future of nanomedicine: anisotropy in polymeric nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 8, 191–207 (2016).
Zhang, L., Chen, Q., Ma, Y. & Sun, J. Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl. Bio Mater. 3, 107–120 (2019).
Hasani-Sadrabadi, M. M. et al. Morphological tuning of polymeric nanoparticles via microfluidic platform for fuel cell applications. J. Am. Chem. Soc. 134, 18904–18907 (2012).
Angly, J. et al. Microfluidic-induced growth and shape-up of three-dimensional extended arrays of densely packed nanoparticles. ACS Nano 7, 6465–6477 (2013).
Sun, X. T. et al. Microfluidic preparation of polymer-lipid Janus microparticles with staged drug release property. J. Colloid Interface Sci. 553, 631–638 (2019).
Xie, H., She, Z.-G., Wang, S., Sharma, G. & Smith, J. W. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 28, 4459–4463 (2012).
Hao, N., Nie, Y., Tadimety, A., Closson, A. B. & Zhang, J. X. J. Microfluidics-mediated self-template synthesis of anisotropic hollow ellipsoidal mesoporous silica nanomaterials. Mater. Res. Lett. 5, 584–590 (2017).
Palagi, S. & Fischer, P. Bioinspired microrobots. Nat. Rev. Mater. 3, 113–124 (2018).
Zhang, Y. & Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 5, 500–510 (2021).
Saper, G. & Hess, H. Synthetic systems powered by biological molecular motors. Chem. Rev. 120, 288–309 (2020).
Soong, R. K. et al. Powering an inorganic nanodevice with a biomolecular motor. Science 290, 1555–1558 (2000).
Tu, Y., Peng, F. & Wilson, D. A. Motion manipulation of micro‐ and nanomotors. Adv. Mater. 29, 1701970 (2017).
Peng, F., Tu, Y. & Wilson, D. A. Micro/nanomotors towards in vivo application: cell, tissue and biofluid. Chem. Soc. Rev. 46, 5289–5310 (2017).
Li, N. et al. Chemotactic NO/H2S nanomotors realizing cardiac targeting of G-CSF against myocardial ischemia-reperfusion injury. ACS Nano 17, 12573–12593 (2023).
Peng, F., Tu, Y., van Hest, J. C. & Wilson, D. A. Self-guided supramolecular cargo-loaded nanomotors with chemotactic behavior towards cells. Angew. Chem. Int. Ed. 54, 11662–11665 (2015).
Liu, X. et al. Enzyme-powered hollow nanorobots for active microsampling enabled by thermoresponsive polymer gating. ACS Nano 16, 10354–10363 (2022).
Wu, Z. et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci. Adv. 4, eaat4388 (2018).
Schattling, P. S., Ramos-Docampo, M. A., Salgueiriño, V. & Städler, B. Double-fueled Janus swimmers with magnetotactic behavior. ACS Nano 11, 3973–3983 (2017).
Abdelmohsen, L. K. et al. Dynamic loading and unloading of proteins in polymeric stomatocytes: formation of an enzyme-loaded supramolecular nanomotor. ACS Nano 10, 2652–2660 (2016).
Wang, L. et al. Continuous microfluidic self-assembly of hybrid Janus-like vesicular motors: autonomous propulsion and controlled release. Small 11, 3762–3767 (2015).
Archer, R. A. et al. pH‐responsive catalytic Janus motors with autonomous navigation and cargo‐release functions. Adv. Funct. Mater. 30, 2000324 (2020).
Díez, P. et al. Ultrafast directional Janus Pt-mesoporous silica nanomotors for smart drug delivery. ACS Nano 15, 4467–4480 (2021).
Wu, Z. et al. Water-powered cell-mimicking Janus micromotor. Adv. Funct. Mater. 25, 7497–7501 (2015).
Gao, W. et al. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano 9, 117–123 (2015).
Peng, F. et al. A peptide functionalized nanomotor as an efficient cell penetrating tool. Chem. Comm. 53, 1088–1091 (2017).
Tu, Y. et al. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 11, 1957–1963 (2017).
Pijpers, I. A. B. et al. Hybrid biodegradable nanomotors through compartmentalized synthesis. Nano Lett. 20, 4472–4480 (2020).
Nance, E. A. et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl Med. 4, 149ra119 (2012).
Ma, X., Hortelão, A. C., Patiño, T. & Sánchez, S. Enzyme catalysis to power micro/nanomachines. ACS Nano 10, 9111–9122 (2016).
Ma, X. et al. Enzyme-powered hollow mesoporous Janus nanomotors. Nano Lett. 15, 7043–7050 (2015).
Rucinskaite, G., Thompson, S. A., Paterson, S. & de la Rica, R. Enzyme-coated Janus nanoparticles that selectively bind cell receptors as a function of the concentration of glucose. Nanoscale 9, 5404–5407 (2017).
Toebes, B. J., Cao, F. & Wilson, D. A. Spatial control over catalyst positioning on biodegradable polymeric nanomotors. Nat. Commun. 10, 5308 (2019).
Wang, L., Hortelão, A. C., Huang, X. & Sánchez, S. Lipase-powered mesoporous silica nanomotors for triglyceride degradation. Angew. Chem. Int. Ed. 58, 7992–7996 (2019).
Fu, D., Ye, Y., Gao, C., Xie, D. & Peng, F. Bienzymatic spiky Janus nanomotors powered by histamine. ChemNanoMat 8, e202200152 (2022).
Hu, Y., Li, Z. & Sun, Y. Ultrasmall enzyme/light-powered nanomotor facilitates cholesterol detection. J. Colloid Interface Sci. 621, 341–351 (2022).
Patiño, T. et al. Influence of enzyme quantity and distribution on the self-propulsion of non-Janus urease-powered micromotors. J. Am. Chem. Soc. 140, 7896–7903 (2018).
Llopis-Lorente, A. et al. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery. ACS Nano 13, 12171–12183 (2019).
Arque, X. et al. Autonomous treatment of bacterial infections in vivo using antimicrobial micro- and nanomotors. ACS Nano 16, 7547–7558 (2022).
Hortelão, A. C., Carrascosa, R., Murillo-Cremaes, N., Patiño, T. & Sánchez, S. Targeting 3D bladder cancer spheroids with urease-powered nanomotors. ACS Nano 13, 429–439 (2019).
Valles, M., Pujals, S., Albertazzi, L. & Sánchez, S. Enzyme purification improves the enzyme loading, self-propulsion, and endurance performance of micromotors. ACS Nano 16, 5615–5626 (2022).
Hao, L.-W. et al. Microfluidic-directed magnetic controlling supraballs with multi-responsive anisotropic photonic crystal structures. J. Mater. Sci. Technol. 81, 203–211 (2021).
Zhang, B. et al. Twin-bioengine self-adaptive micro/nanorobots using enzyme actuation and macrophage relay for gastrointestinal inflammation therapy. Sci. Adv. 9, eadc8978 (2023).
Wu, Z. et al. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew. Chem. Int. Ed. 52, 7000–7003 (2013).
Wan, M. et al. Bio-inspired nitric-oxide-driven nanomotor. Nat. Commun. 10, 966 (2019).
Wu, Z. et al. Carrier-free trehalose-based nanomotors targeting macrophages in inflammatory plaque for treatment of atherosclerosis. ACS Nano 16, 3808–3820 (2022).
Li, J., Mayorga‐Martinez, C. C., Ohl, C. D. & Pumera, M. Ultrasonically propelled micro‐ and nanorobots. Adv. Funct. Mater. 32, 2102265 (2022).
Garcia-Gradilla, V. et al. Functionalized ultrasound-propelled magnetically guided nanomotors: toward practical biomedical applications. ACS Nano 7, 9232–9240 (2013).
Garcia-Gradilla, V. et al. Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small 10, 4154–4159 (2014).
Esteban-Fernández de Ávila, B. et al. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano 10, 4997–5005 (2016).
Cardoso, V. F. et al. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 7, 1700845 (2018).
Gao, W., Sattayasamitsathit, S., Manesh, K. M., Weihs, D. & Wang, J. Magnetically powered flexible metal nanowire motors. J. Am. Chem. Soc. 132, 14403–14405 (2010).
Schamel, D. et al. Nanopropellers and their actuation in complex viscoelastic media. ACS Nano 8, 8794–8801 (2014).
Ghosh, A., Paria, D., Rangarajan, G. & Ghosh, A. Velocity fluctuations in helical propulsion: how small can a propeller be. J. Phys. Chem. Lett. 5, 62–68 (2014).
Venugopalan, P. L., Jain, S., Shivashankar, S. & Ghosh, A. Single coating of zinc ferrite renders magnetic nanomotors therapeutic and stable against agglomeration. Nanoscale 10, 2327–2332 (2018).
Venugopalan, P. L. et al. Conformal cytocompatible ferrite coatings facilitate the realization of a nanovoyager in human blood. Nano Lett. 14, 1968–1975 (2014).
Ramachandran, R. V. et al. How safe are magnetic nanomotors: from cells to animals. Bio. Adv. 140, 213048 (2022).
Shen, Y. et al. Adaptive control of nanomotor swarms for magnetic-field-programmed cancer cell destruction. ACS Nano 15, 20020–20031 (2021).
Xu, L., Mou, F., Gong, H., Luo, M. & Guan, J. Light-driven micro/nanomotors: from fundamentals to applications. Chem. Soc. Rev. 46, 6905–6926 (2017).
Shao, J. et al. Erythrocyte membrane modified Janus polymeric motors for thrombus therapy. ACS Nano 12, 4877–4885 (2018).
Peng, X. et al. Opto-thermoelectric microswimmers. Light Sci. Appl. 9, 141 (2020).
Sridhar, V. et al. Carbon nitride-based light-driven microswimmers with intrinsic photocharging ability. Proc. Natl Acad. Sci. USA 117, 24748–24756 (2020).
Cao, S. et al. Photoactivated nanomotors via aggregation induced emission for enhanced phototherapy. Nat. Commun. 12, 2077 (2021).
Chen, S. et al. Dual-source powered nanomotor with integrated functions for cancer photo-theranostics. Biomaterials 288, 121744 (2022).
Zheng, S. et al. Biocompatible nanomotors as active diagnostic imaging agents for enhanced magnetic resonance imaging of tumor tissues in vivo. Adv. Funct. Mater. 31, 2100936 (2021).
Liu, J. et al. Rotary biomolecular motor-powered supramolecular colloidal motor. Sci. Adv. 9, eabg3015 (2023).
Zhou, D. et al. Light-ultrasound driven collective “firework” behavior of nanomotors. Adv. Sci. 5, 1800122 (2018).
Li, J. et al. Magneto-acoustic hybrid nanomotor. Nano Lett. 15, 4814–4821 (2015).
Shao, J. et al. Twin-engine Janus supramolecular nanomotors with counterbalanced motion. J. Am. Chem. Soc. 144, 11246–11252 (2022).
Yu, M. et al. Rotation-facilitated rapid transport of nanorods in mucosal tissues. Nano Lett. 16, 7176–7182 (2016).
Guo, M. et al. Impacts of particle shapes on the oral delivery of drug nanocrystals: mucus permeation, transepithelial transport and bioavailability. J. Control. Release 307, 64–75 (2019).
Bao, C. et al. Enhanced transport of shape and rigidity-tuned α-lactalbumin nanotubes across intestinal mucus and cellular barriers. Nano Lett. 20, 1352–1361 (2020).
Sosnik, A., das Neves, J. & Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog. Polym. Sci. 39, 2030–2075 (2014).
Wang, Z. H. et al. Self-thermophoretic nanoparticles enhance intestinal mucus penetration and reduce pathogenic bacteria interception in colorectal cancer. Adv. Funct. Mater. 33, 2212013 (2023).
Geng, Y. et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2, 249–255 (2007).
Xiong, F. et al. Superparamagnetic anisotropic nano-assemblies with longer blood circulation in vivo: a highly efficient drug delivery carrier for leukemia therapy. Nanoscale 8, 17085–17089 (2016).
Zhou, Z. et al. Linear-dendritic drug conjugates forming long circulating nanorods for cancer-drug delivery. Biomaterials 34, 5722–5735 (2013).
Kapate, N., Clegg, J. R. & Mitragotri, S. Non-spherical micro- and nanoparticles for drug delivery: progress over 15 years. Adv. Drug Deliv. Rev. 177, 113807 (2021).
van de Ven, A. L. et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Control. Release 158, 148–155 (2012).
Jurney, P. et al. Unique size and shape-dependent uptake behaviors of non-spherical nanoparticles by endothelial cells due to a shearing flow. J. Control. Release 245, 170–176 (2017).
Cooley, M. et al. Influence of particle size and shape on their margination and wall-adhesion: implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 10, 15350–15364 (2018).
Gupta, R., Badhe, Y., Mitragotri, S. & Rai, B. Permeation of nanoparticles across the intestinal lipid membrane: dependence on shape and surface chemistry studied through molecular simulations. Nanoscale 12, 6318–6333 (2020).
Sikder, A., Pearce, A. K., Kumar, C. M. S. & O’Reilly, R. K. Elucidating the role of multivalency, shape, size and functional group density on antibacterial activity of diversified supramolecular nanostructures enabled by templated assembly. Mater. Horiz. 10, 171–178 (2023).
Anselmo, A. C. et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8, 11243–11253 (2014).
Barua, S. et al. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl Acad. Sci. USA 110, 3270–3275 (2013).
Wong, S. H. D. et al. Anisotropic nanoscale presentation of cell adhesion ligand enhances the recruitment of diverse integrins in adhesion structures and mechanosensing-dependent differentiation of stem cells. Adv. Funct. Mater. 29, 1806822 (2019).
Da Silva-Candal, A. et al. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J. Control. Release 309, 94–105 (2019).
Acter, S., Vidallon, M. L. P., Crawford, S., Tabor, R. F. & Teo, B. M. Bowl-shaped mesoporous polydopamine nanoparticles for size-dependent endocytosis into HeLa cells. ACS Appl. Nano Mater. 4, 9536–9546 (2021).
Acter, S., Vidallon, M. L. P., Crawford, S., Tabor, R. F. & Teo, B. M. Efficient cellular internalization and transport of bowl‐shaped polydopamine particles. Part. Part. Syst. Charact. 37, 2000166 (2020).
Talamini, L. et al. Influence of size and shape on the anatomical distribution of endotoxin-free gold nanoparticles. ACS Nano 11, 5519–5529 (2017).
García-Álvarez, R., Hadjidemetriou, M., Sánchez-Iglesias, A., Liz-Marzán, L. M. & Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 10, 1256–1264 (2018).
Joseph, A. et al. Chemotactic synthetic vesicles: design and applications in blood-brain barrier crossing. Sci. Adv. 3, e1700362 (2017).
Brown, T. D., Habibi, N., Wu, D., Lahann, J. & Mitragotri, S. Effect of nanoparticle composition, size, shape, and stiffness on penetration across the blood-brain barrier. ACS Biomater. Sci. Eng. 6, 4916–4928 (2020).
Kolhar, P. et al. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl Acad. Sci. USA 110, 10753–10758 (2013).
Nowak, M., Brown, T. D., Graham, A., Helgeson, M. E. & Mitragotri, S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng. Transl. Med. 5, e10153 (2020).
Han, S. et al. Spatiotemporal tracking of gold nanorods after intranasal administration for brain targeting. J. Control. Release 357, 606–619 (2023).
Ju, Y. et al. Monodisperse Au-Fe2C Janus nanoparticles: an attractive multifunctional material for triple-modal imaging-guided tumor photothermal therapy. ACS Nano 11, 9239–9248 (2017).
Deng, Q. et al. Biological mediator-propelled nanosweeper for nonpharmaceutical thrombus therapy. ACS Nano 15, 6604–6613 (2021).
Xie, S. et al. Self-propelling nanomotors integrated with biofilm microenvironment-activated NO release to accelerate healing of bacteria-infected diabetic wounds. Adv. Healthc. Mater. 11, e2201323 (2022).
Wibroe, P. P. et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 12, 589–594 (2017).
Kumar, S., Anselmo, A. C., Banerjee, A., Zakrewsky, M. & Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 220, 141–148 (2015).
Shukla, S. et al. The impact of aspect ratio on the biodistribution and tumor homing of rigid soft-matter nanorods. Adv. Healthc. Mater. 4, 874–882 (2015).
Li, Z. et al. Shape effect of glyco-nanoparticles on macrophage cellular uptake and immune response. ACS Macro Lett. 5, 1059–1064 (2016).
Meyer, R. A. et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 11, 1519–1525 (2015).
Hassani Najafabadi, A. et al. Cancer immunotherapy via targeting cancer stem cells using vaccine nanodiscs. Nano Lett. 20, 7783–7792 (2020).
Kuai, R. et al. Robust anti-tumor T cell response with efficient intratumoral infiltration by nanodisc cancer immunotherapy. Adv. Ther. 3, 2000094 (2020).
Kadiyala, P. et al. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano 13, 1365–1384 (2019).
Scheetz, L. et al. Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas. Clin. Cancer Res. 26, 4369–4380 (2020).
Tazaki, T. et al. Shape-dependent adjuvanticity of nanoparticle-conjugated RNA adjuvants for intranasal inactivated influenza vaccines. RSC Adv. 8, 16527–16536 (2018).
Wang, Z. et al. Fluidity-guided assembly of Au@Pt on liposomes as a catalase-powered nanomotor for effective cell uptake in cancer cells and plant leaves. ACS Nano 16, 9019–9030 (2022).
Ou, J. et al. MnO2-based nanomotors with active Fenton-like Mn2+ delivery for enhanced chemodynamic therapy. ACS Appl. Mater. Interfaces 13, 38050–38060 (2021).
Yang, Z. et al. Ultrasmall enzyme-powered Janus nanomotor working in blood circulation system. ACS Nano 17, 6023–6035 (2023).
Choi, H., Cho, S. H. & Hahn, S. K. Urease-powered polydopamine nanomotors for intravesical therapy of bladder diseases. ACS Nano 14, 6683–6692 (2020).
Tong, F. et al. Carbon monoxide-propelled nanomotors as an active treatment for renal injury. Appl. Mater. Today 32, 101823 (2023).
Kiristi, M. et al. Lysozyme-based antibacterial nanomotors. ACS Nano 9, 9252–9259 (2015).
Hansen-Bruhn, M. et al. Active intracellular delivery of a Cas9/sgRNA complex using ultrasound-propelled nanomotors. Angew. Chem. Int. Ed. 57, 2657–2661 (2018).
Wang, W. et al. Acoustic propulsion of nanorod motors inside living cells. Angew. Chem. Int. Ed. 53, 3201–3204 (2014).
Pal, M. et al. Maneuverability of magnetic nanomotors inside living cells. Adv. Mater. 30, e1800429 (2018).
Zhang, X. et al. NIR-propelled Janus nanomotors for active photoacoustic imaging and synergistic photothermal/chemodynamic therapy. J. Colloid Interface Sci. 648, 457–472 (2023).
Meng, J. et al. Pyroelectric Janus nanomotors to promote cell internalization and synergistic tumor therapy. J. Control. Release 357, 342–355 (2023).
Liu, Y. et al. NIR-II-activated yolk-shell nanostructures as an intelligent platform for Parkinsonian therapy. ACS Appl. Bio Mater. 3, 6876–6887 (2020).
Jarvis, M., Krishnan, V. & Mitragotri, S. Nanocrystals: a perspective on translational research and clinical studies. Bioeng. Transl. Med. 4, 5–16 (2019).
Jahn, M. R. et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 78, 480–491 (2011).
Takahashi, N., Higashi, K., Ueda, K., Yamamoto, K. & Moribe, K. Determination of nonspherical morphology of doxorubicin-loaded liposomes by atomic force microscopy. J. Pharm. Sci. 107, 717–726 (2018).
Ekanem, E. E., Zhang, Z. & Vladisavljevic, G. T. Facile production of biodegradable bipolymer patchy and patchy Janus particles with controlled morphology by microfluidic routes. Langmuir 33, 8476–8482 (2017).
Cao, X., Li, W., Ma, T. & Dong, H. One-step fabrication of polymeric hybrid particles with core–shell, patchy, patchy Janus and Janus architectures via a microfluidic-assisted phase separation process. RSC Adv. 5, 79969–79975 (2015).
Du, J. & O’Reilly, R. K. Anisotropic particles with patchy, multicompartment and Janus architectures: preparation and application. Chem. Soc. Rev. 40, 2402–2416 (2011).
Walther, A. & Muller, A. H. Janus particles: synthesis, self-assembly, physical properties, and applications. Chem. Rev. 113, 5194–5261 (2013).
Lesov, I. et al. Bottom-up synthesis of polymeric micro- and nanoparticles with regular anisotropic shapes. Macromolecules 51, 7456–7462 (2018).
Verhoef, J. J. F. et al. Iron nanomedicines induce Toll-like receptor activation, cytokine production and complement activation. Biomaterials 119, 68–77 (2017).
Scott, L. J. Ferric carboxymaltose: a review in iron deficiency. Drugs 78, 479–493 (2018).
Acknowledgements
H.A. and J.d.N. gratefully acknowledge Fundação para a Ciência e a Tecnologia, Portugal, for financial support (2020.06264.BD fellowship and CEECIND/01280/2018 contract under the Individual CEEC Program, respectively).
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conceptualization of the article, data search and discussion of content. H.A. wrote the initial draft of the manuscript. G.T., B.S. and J.d.N. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
Financial competing interests for G.T. that may be interpreted as related to the current manuscript include current and prior funding (Supplementary Tables 2 and 3) from Novo Nordisk, CSL Vifor, Hoffman La Roche, Oracle, Draper Laboratory, Massachusetts Institute of Technology (MIT) Lincoln Laboratory, National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering, National Cancer Institute, Advanced Research Projects Agency for Health), Bill and Melinda Gates Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, Karl van Tassel (1925) Career Development Professor, MIT and the Defense Advanced Research Projects Agency, as well as employment by the MIT and Brigham and Women’s Hospital (Supplementary Table 1). Personal financial interests include equity/stock (Lyndra Therapeutics, Suono Bio, Vivtex, Celero Systems, Syntis Bio), board of directors membership and/or consulting (Lyndra Therapeutics, Novo Nordisk, Suono Bio, Vivtex, Celero Systems, Syntis Bio) and royalties (past and potentially in the future) from licensed and/or optioned intellectual property (Lyndra Therapeutics, Novo Nordisk, Suono Bio, Vivtex, Celero Systems, Syntis Bio, Johns Hopkins, MIT, and Mass General Brigham Innovation). Complete details of all relationships for-profit and not-for-profit for G.T. can be found in the Supplementary Information. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Takuro Niidome and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almeida, H., Traverso, G., Sarmento, B. et al. Nanoscale anisotropy for biomedical applications. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00169-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s44222-024-00169-2