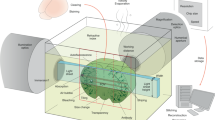
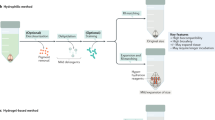
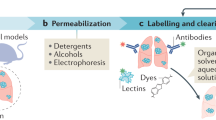
Abstract
Tissue clearing of gross anatomical samples was first described more than a century ago and has only recently found widespread use in the field of microscopy. This renaissance has been driven by the application of modern knowledge of optical physics and chemical engineering to the development of robust and reproducible clearing techniques, the arrival of new microscopes that can image large samples at cellular resolution and computing infrastructure able to store and analyse large volumes of data. Many biological relationships between structure and function require investigation in three dimensions, and tissue clearing therefore has the potential to enable broad discoveries in the biological sciences. Unfortunately, the current literature is complex and could confuse researchers looking to begin a clearing project. The goal of this Primer is to outline a modular approach to tissue clearing that allows a novice researcher to develop a customized clearing pipeline tailored to their tissue of interest. Furthermore, the Primer outlines the required imaging and computational infrastructure needed to perform tissue clearing at scale, gives an overview of current applications, discusses limitations and provides an outlook on future advances in the field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Richardson, D. S. & Lichtman, J. W. Clarifying tissue clearing. Cell 162, 246–257 (2015).
Tainaka, K., Kuno, A., Kubota, S. I., Murakami, T. & Ueda, H. R. Chemical principles in tissue clearing and staining protocols for whole-body cell profiling. Annu. Rev. Cell Dev. Biol. 32, 713–741 (2016).
Ueda, H. R. et al. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 21, 61–79 (2020).
Park, Y.-G. et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. https://doi.org/10.1038/nbt.4281 (2018).
Susaki, E. A. et al. Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nat. Commun. 11, 1982 (2020).
Zhao, S. et al. Cellular and molecular probing of intact human organs. Cell 180, 796–812.e719 (2020).
Kubota, S. I. et al. Whole-body profiling of cancer metastasis with single-cell resolution. Cell Rep. 20, 236–250 (2017).
Lee, E. et al. ACT-PRESTO: rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci. Rep. 6, 18631 (2016).
Pan, C. et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 13, 859–867 (2016).
Susaki, E. A. & Ueda, H. R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: toward organism-level systems biology in mammals. Cell Chem. Biol. 23, 137–157 (2016).
Tainaka, K. et al. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159, 911–924 (2014).
Messal, H. A. et al. Antigen retrieval and clearing for whole-organ immunofluorescence by FLASH. Nat. Protoc. 16, 239–262 (2021).
Pende, M. et al. High-resolution ultramicroscopy of the developing and adult nervous system in optically cleared Drosophila melanogaster. Nat. Commun. 9, 4731 (2018).
Lindsey, B. W., Douek, A. M., Loosli, F. & Kaslin, J. A whole brain staining, embedding, and clearing pipeline for adult zebrafish to visualize cell proliferation and morphology in 3-dimensions. Front. Neurosci. 11, 750 (2017).
Pende, M. et al. A versatile depigmentation, clearing, and labeling method for exploring nervous system diversity. Sci. Adv. 6, eaba0365 (2020).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Menegas, W. et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032 (2015).
Ravindra Kumar, S. et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat. Methods 17, 541–550 (2020).
Hopwood, D. The reactions between formaldehyde, glutaraldehyde and osmium tetroxide, and their fixation effects o bovine serum albumin and on tissue blocks. Histochemie 24, 50–64 (1970).
Murray, E. et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163, 1500–1514 (2015).
Collins, J. S. & Goldsmith, T. H. Spectral properties of fluorescence induced by glutaraldehyde fixation. J. Histochem. Cytochem. 29, 411–414 (1981).
Ueda, H. R. et al. Whole-brain profiling of cells and circuits in mammals by tissue clearing and light-sheet microscopy. Neuron 106, 369–387 (2020).
Susaki, E. A. et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014).
Matsumoto, K. et al. Advanced CUBIC tissue clearing for whole-organ cell profiling. Nat. Protoc. 14, 3506–3537 (2019).
Tainaka, K. et al. Chemical landscape for tissue clearing based on hydrophilic reagents. Cell Rep. 24, 2196–2210 (2018).
Kim, S.-Y. & Assawachananont, J. A new method to visualize the intact subretina from retinal pigment epithelium to retinal tissue in whole mount of pigmented mouse eyes. Transl. Vis. Sci. Technol. 5, 6 (2016).
Futami, K., Furukawa, O., Maita, M. & Katagiri, T. Application of hydrogen peroxide-melanin bleaching and fluorescent nuclear staining for whole-body clearing and imaging in fish. Fish. Pathol. 54, 101–103 (2020).
Kuroda, M. & Kuroda, S. Whole-body clearing of beetles by successive treatment with hydrogen peroxide and CUBIC reagents. Entomol. Sci. 23, 311–315 (2020).
Henning, Y., Osadnik, C. & Malkemper, E. P. EyeCi: optical clearing and imaging of immunolabeled mouse eyes using light-sheet fluorescence microscopy. Exp. Eye Res. 180, 137–145 (2019).
Duong, H. & Han, M. A multispectral LED array for the reduction of background autofluorescence in brain tissue. J. Neurosci. Methods 220, 46–54 (2013).
Ku, T. et al. Elasticizing tissues for reversible shape transformation and accelerated molecular labeling. Nat. Methods 17, 609–613 (2020).
Choi, S. W., Guan, W. & Chung, K. Basic principles of hydrogel-based tissue transformation technologies and their applications. Cell 184, 4115–4136 (2021).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Yang, B. et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945–958 (2014).
Chen, F., Tillberg, P. W. & Boyden, E. S. Optical imaging. expansion microscopy. Science 347, 543–548 (2015).
Tillberg, P. W. et al. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. Nat. Biotechnol. 34, 987–992 (2016).
Ku, T. et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat. Biotechnol. 34, 973–981 (2016).
Murakami, T. C. et al. A three-dimensional single-cell-resolution whole-brain atlas using CUBIC-X expansion microscopy and tissue clearing. Nat. Neurosci. 21, 625–637 (2018).
Chang, J. B. et al. Iterative expansion microscopy. Nat. Methods 14, 593–599 (2017).
Park, H. E. et al. Scalable and isotropic expansion of tissues with simply tunable expansion ratio. Adv. Sci. 6, 1901673 (2019).
Kiviranta, I., Tammi, M., Lappalainen, R., Kuusela, T. & Helminen, H. J. The rate of calcium extraction during EDTA decalcification from thin bone slices as assessed with atomic absorption spectrophotometry. Histochemistry 68, 119–127 (1980).
Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 28, 803–818 (2018).
Treweek, J. B. et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc. 10, 1860–1896 (2015).
Greenbaum, A. et al. Bone CLARITY: clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Sci. Transl. Med. 9, eaah6518 (2017).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Renier, N. et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Dodt, H. U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007).
Qi, Y. et al. FDISCO: advanced solvent-based clearing method for imaging whole organs. Sci. Adv. 5, eaau8355 (2019).
Hahn, C. et al. High-resolution imaging of fluorescent whole mouse brains using stabilised organic media (sDISCO). J. Biophotonics 12, e201800368 (2019).
Schwarz, M. K. et al. Fluorescent-protein stabilization and high-resolution imaging of cleared, intact mouse brains. PLoS ONE 10, e0124650 (2015).
Li, Y., Xu, J., Wan, P., Yu, T. & Zhu, D. Optimization of GFP fluorescence preservation by a modified uDISCO clearing protocol. Front. Neuroanat. 12, 67 (2018).
Becker, K., Jahrling, N., Saghafi, S., Weiler, R. & Dodt, H. U. Chemical clearing and dehydration of GFP expressing mouse brains. PLoS ONE 7, e33916 (2012).
Erturk, A. et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 7, 1983–1995 (2012).
Hama, H. et al. ScaleS: an optical clearing palette for biological imaging. Nat. Neurosci. 18, 1518–1529 (2015).
Chen, L. et al. UbasM: An effective balanced optical clearing method for intact biomedical imaging. Sci. Rep. 7, 12218 (2017).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27, 226–236 (2018).
Kim, S. Y. et al. Stochastic electrotransport selectively enhances the transport of highly electromobile molecules. Proc. Natl Acad. Sci. USA 112, E6274–E6283 (2015).
Hoelzel, C. A. & Zhang, X. Visualizing and manipulating biological processes by using halotag and SNAP-tag technologies. ChemBioChem 21, 1935–1946 (2020).
Fang, T. et al. Nanobody immunostaining for correlated light and electron microscopy with preservation of ultrastructure. Nat. Methods 15, 1029–1032 (2018).
Burry, R. W. Immunocytochemistry: A Practical Guide for Biomedical Research (Springer, 2010).
Sahl, S. J., Hell, S. W. & Jakobs, S. Fluorescence nanoscopy in cell biology. Nat. Rev. Mol. Cell Biol. 18, 685–701 (2017).
Muyldermans, S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 82, 775–797 (2013).
Lai, H. M. et al. Next generation histology methods for three-dimensional imaging of fresh and archival human brain tissues. Nat. Commun. 9, 1066 (2018).
Gleave, J. A., Lerch, J. P., Henkelman, R. M. & Nieman, B. J. A method for 3D immunostaining and optical imaging of the mouse brain demonstrated in neural progenitor cells. PLoS ONE 8, e72039 (2013).
Kumar, V. et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell–lymphotoxin-dependent pathway. Blood 115, 4725–4733 (2010).
Sillitoe, R. V. & Hawkes, R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 50, 235–244 (2002).
Na, M., Kim, K., Lim, H. R., Ha, C. M. & Chang, S. Rapid immunostaining method for three-dimensional volume imaging of biological tissues by magnetic force-induced focusing of the electric field. Brain Struct. Funct. 226, 297–309 (2021).
Dwyer, J., Ramirez, M. D., Katz, P. S., Karlstrom, R. O. & Bergan, J. Accelerated clearing and molecular labeling of biological tissues using magnetohydrodynamic force. Sci. Rep. 11, 16462 (2021).
Takahashi, K., Kubota, S. I., Ehata, S., Ueda, H. R. & Miyazono, K. Protocol for imaging and analysis of mouse tumor models with CUBIC tissue clearing. STAR. Protoc. 1, 100191 (2020).
Li, W., Germain, R. N. & Gerner, M. Y. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc. Natl Acad. Sci. USA 114, E7321–E7330 (2017).
Klingberg, A. et al. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 28, 452–459 (2017).
Aoyagi, Y., Kawakami, R., Osanai, H., Hibi, T. & Nemoto, T. A rapid optical clearing protocol using 2,2′-thiodiethanol for microscopic observation of fixed mouse brain. PLoS ONE 10, e0116280 (2015).
Costantini, I. et al. A versatile clearing agent for multi-modal brain imaging. Sci. Rep. 5, 9808 (2015).
Staudt, T., Lang, M. C., Medda, R., Engelhardt, J. & Hell, S. W. 2,2′-Thiodiethanol: a new water soluble mounting medium for high resolution optical microscopy. Microscopy Res. Tech. 70, 1–9 (2007).
Diel, E. E., Lichtman, J. W. & Richardson, D. S. Tutorial: avoiding and correcting sample-induced spherical aberration artifacts in 3D fluorescence microscopy. Nat. Protoc. 15, 2773–2784 (2020).
Vallejo Ramirez, P. P. et al. OptiJ: open-source optical projection tomography of large organ samples. Sci. Rep. 9, 15693 (2019).
Mayer, J. et al. OPTiSPIM: integrating optical projection tomography in light sheet microscopy extends specimen characterization to nonfluorescent contrasts. Opt. Lett. 39, 1053–1056 (2014).
Baek, K. et al. Quantitative assessment of regional variation in tissue clearing efficiency using optical coherence tomography (OCT) and magnetic resonance imaging (MRI): a feasibility study. Sci. Rep. 9, 2923 (2019).
Pietzsch, T., Saalfeld, S., Preibisch, S. & Tomancak, P. BigDataViewer: visualization and processing for large image data sets. Nat. Methods 12, 481–483 (2015).
Jonkman, J., Brown, C. M., Wright, G. D., Anderson, K. I. & North, A. J. Tutorial: guidance for quantitative confocal microscopy. Nat. Protoc. 15, 1585–1611 (2020).
Jost, A. P. & Waters, J. C. Designing a rigorous microscopy experiment: validating methods and avoiding bias. J. Cell Biol. 218, 1452–1466 (2019).
Horl, D. et al. BigStitcher: reconstructing high-resolution image datasets of cleared and expanded samples. Nat. Methods 16, 870–874 (2019).
Prahst, C. et al. Mouse retinal cell behaviour in space and time using light sheet fluorescence microscopy. eLife 9, e49779 (2020).
Moen, E. et al. Deep learning for cellular image analysis. Nat. Methods 16, 1233–1246 (2019).
Pan, C. et al. Deep learning reveals cancer metastasis and therapeutic antibody targeting in the entire body. Cell 179, 1661–1676 (2019).
Schmidt, U., Weigert, M., Broaddus, C. & Myers, G. in Medical Image Computing and Computer Assisted Intervention – MICCAI 2018 (eds Frangi, A. F., Schnabel, J. A., Davatzikos, C., Alberola-López, C. & Fichtinger, G.) 265–273 (Springer, 2018).
Todorov, M. I. et al. Machine learning analysis of whole mouse brain vasculature. Nat. Methods 17, 442–449 (2020).
Qi, Y. in Ensemble Machine Learning: Methods and Applications (eds Zhang, C. & Ma, Y.) 307–323 (Springer, 2012).
Dhillon, A. & Verma, G. K. Convolutional neural network: a review of models, methodologies and applications to object detection. Prog. Artif. Intell. 9, 85–112 (2020).
Callara, A. L., Magliaro, C., Ahluwalia, A. & Vanello, N. A smart region-growing algorithm for single-neuron segmentation from confocal and 2-photon datasets. Front. Neuroinform. 14, 9 (2020).
Richardson, D. S. et al. SRpHi ratiometric pH biosensors for super-resolution microscopy. Nat. Commun. 8, 577 (2017).
Gao, R. et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 363, eaau8302 (2019).
Randlett, O. et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat. Methods 12, 1039–1046 (2015).
Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012).
Mikula, S., Trotts, I., Stone, J. M. & Jones, E. G. Internet-enabled high-resolution brain mapping and virtual microscopy. NeuroImage 35, 9–15 (2007).
Amunts, K. et al. BigBrain: an ultrahigh-resolution 3D human brain model. Science 340, 1472–1475 (2013).
Calabrese, E. et al. A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. NeuroImage 117, 408–416 (2015).
Rohlfing, T. et al. The INIA19 template and neuromaps atlas for primate brain image parcellation and spatial normalization. Front. Neuroinform. 6, 27 (2012).
Johnson, G. A. et al. Waxholm space: an image-based reference for coordinating mouse brain research. NeuroImage 53, 365–372 (2010).
Papp, E. A., Leergaard, T. B., Calabrese, E., Johnson, G. A. & Bjaalie, J. G. Waxholm space atlas of the sprague dawley rat brain. NeuroImage 97, 374–386 (2014).
Dong, H. W. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57Bl/6J Male Mouse (John Wiley & Sons, Inc., 2008).
Kuan, L. et al. Neuroinformatics of the allen mouse brain connectivity atlas. Methods 73, 4–17 (2015).
Mano, T. et al. CUBIC-Cloud provides an integrative computational framework toward community-driven whole-mouse-brain mapping. Cell Rep. Methods 1, 100038 (2021).
Ye, L. et al. Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell 165, 1776–1788 (2016).
Kutten, K. S. A. V. et al. in Optics, Photonics and Digital Technologies for Imaging Applications IV (SPIE, 2016).
Gradinaru, V., Treweek, J., Overton, K. & Deisseroth, K. Hydrogel-tissue chemistry: principles and applications. Annu. Rev. Biophys. 47, 355–376 (2018).
Baiker, M. et al. Atlas-based whole-body segmentation of mice from low-contrast micro-CT data. Med. Image Anal. 14, 723–737 (2010).
Dogdas, B., Stout, D., Chatziioannou, A. F. & Leahy, R. M. Digimouse: a 3D whole body mouse atlas from CT and cryosection data. Phys. Med. Biol. 52, 577–587 (2007).
Schoppe, O. et al. Deep learning-enabled multi-organ segmentation in whole-body mouse scans. Nat. Commun. 11, 5626 (2020).
Haase, R. et al. CLIJ: GPU-accelerated image processing for everyone. Nat. Methods 17, 5–6 (2020).
Kirst, C. et al. Mapping the fine-scale organization and plasticity of the brain vasculature. Cell 180, 780–795 (2020).
Hahn, M. et al. 3D imaging of human organs with micrometer resolution — applied to the endocrine pancreas. Commun. Biol. 4, 1063 (2021).
Zhao, Y. et al. Nanoscale imaging of clinical specimens using pathology-optimized expansion microscopy. Nat. Biotechnol. 35, 757–764 (2017).
Campinho, M. A. et al. A thyroid hormone regulated asymmetric responsive centre is correlated with eye migration during flatfish metamorphosis. Sci. Rep. 8, 12267 (2018).
Konno, A. & Okazaki, S. Aqueous-based tissue clearing in crustaceans. Zool. Lett. 4, 13 (2018).
Albanese, A. et al. Multiscale 3D phenotyping of human cerebral organoids. Sci. Rep. 10, 21487 (2020).
Winnubst, J. et al. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 179, 268–281 (2019).
Weissbourd, B. et al. Functional modules within a distributed neural network control feeding in a model medusa. Preprint at bioRxiv https://doi.org/10.1101/2021.02.22.432372 (2021).
Morgan, J. L. & Lichtman, J. W. Digital tissue and what it may reveal about the brain. BMC Biol. 15, 101 (2017).
Economo, M. N., Winnubst, J., Bas, E., Ferreira, T. A. & Chandrashekar, J. Single-neuron axonal reconstruction: the search for a wiring diagram of the brain. J. Comp. Neurol. 527, 2190–2199 (2019).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Cai, D., Cohen, K. B., Luo, T., Lichtman, J. W. & Sanes, J. R. Improved tools for the Brainbow toolbox. Nat. Methods 10, 540–547 (2013).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Quan, T. et al. NeuroGPS-Tree: automatic reconstruction of large-scale neuronal populations with dense neurites. Nat. Methods 13, 51–54 (2016).
Wang, X. et al. Bi-channel image registration and deep-learning segmentation (BIRDS) for efficient, versatile 3D mapping of mouse brain. eLife 10, e63455 (2021).
Gail Canter, R. et al. 3D mapping reveals network-specific amyloid progression and subcortical susceptibility in mice. Commun. Biol. 2, 360 (2019).
Sabdyusheva Litschauer, I. et al. 3D histopathology of human tumours by fast clearing and ultramicroscopy. Sci. Rep. 10, 17619 (2020).
Ma, Y. et al. 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv. Mater. 30, 1705911 (2018).
Lloyd-Lewis, B. Multidimensional imaging of mammary gland development: a window into breast form and function. Front. Cell Dev. Biol. 8, 203 (2020).
Yang, L. et al. Three-dimensional quantitative co-mapping of pulmonary morphology and nanoparticle distribution with cellular resolution in nondissected murine lungs. ACS Nano 13, 1029–1041 (2019).
Cuccarese, M. F. et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 8, 14293 (2017).
Sindhwani, S., Syed, A. M., Wilhelm, S. & Chan, W. C. Exploring passive clearing for 3d optical imaging of nanoparticles in intact tissues. Bioconjug Chem. 28, 253–259 (2017).
Schimmenti, L. A., Yan, H. C., Madri, J. A. & Albelda, S. M. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J. Cell Physiol. 153, 417–428 (1992).
Konno, A., Matsumoto, N. & Okazaki, S. Improved vessel painting with carbocyanine dye-liposome solution for visualisation of vasculature. Sci. Rep. 7, 10089 (2017).
Nehrhoff, I., Ripoll, J., Samaniego, R., Desco, M. & Gomez-Gaviro, M. V. Looking inside the heart: a see-through view of the vascular tree. Biomed. Opt. Express 8, 3110–3118 (2017).
Lugo-Hernandez, E. et al. 3D visualization and quantification of microvessels in the whole ischemic mouse brain using solvent-based clearing and light sheet microscopy. J. Cereb. Blood Flow. Metab. 37, 3355–3367 (2017).
Di Giovanna, A. P. et al. Whole-brain vasculature reconstruction at the single capillary level. Sci. Rep. 8, 12573 (2018).
Rajendran, P. S. et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat. Commun. 10, 1944 (2019).
Achanta, S. et al. A comprehensive integrated anatomical and molecular atlas of rat intrinsic cardiac nervous system. iScience 23, 101140 (2020).
Yokoyama, T. et al. Quantification of sympathetic hyperinnervation and denervation after myocardial infarction by three-dimensional assessment of the cardiac sympathetic network in cleared transparent murine hearts. PLoS ONE 12, e0182072 (2017).
Kapp, F. G. et al. Protection from UV light is an evolutionarily conserved feature of the haematopoietic niche. Nature 558, 445–448 (2018).
Lersten, N. R. Modified clearing method to show sieve tubes in minor veins of leaves. Stain Technol. 61, 231–234 (1986).
Warner, C. A. et al. An optical clearing technique for plant tissues allowing deep imaging and compatible with fluorescence microscopy. Plant. Physiol. 166, 1684–1687 (2014).
Kurihara, D., Mizuta, Y., Sato, Y. & Higashiyama, T. ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168–4179 (2015).
Lu, L. et al. A rapid and effective optical-clearing technique for deep tissue fluorescence imaging in trees. Trees Struct. Funct. 34, 783–790 (2020).
Xia, Q. et al. Solar-assisted fabrication of large-scale, patternable transparent wood. Sci. Adv. 7, eabd7342 (2021).
Nojima, S. et al. CUBIC pathology: three-dimensional imaging for pathological diagnosis. Sci. Rep. 7, 9269 (2017).
Belle, M. et al. Tridimensional visualization and analysis of early human development. Cell 169, 161–173 (2017).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Rigamonti, A. et al. Large-scale production of mature neurons from human pluripotent stem cells in a three-dimensional suspension culture system. Stem Cell Rep. 6, 993–1008 (2016).
Cora, V. et al. A cleared view on retinal organoids. Cells 8, 391 (2019).
Costantini, I., Cicchi, R., Silvestri, L., Vanzi, F. & Pavone, F. S. In-vivo and ex-vivo optical clearing methods for biological tissues: review. Biomed. Opt. Express 10, 5251–5267 (2019).
Deng, Z. J. et al. Viscous optical clearing agent for in vivo optical imaging. J. Biomed. Opt. 19, 76019 (2014).
Millon, S. R., Roldan-Perez, K. M., Riching, K. M., Palmer, G. M. & Ramanujam, N. Effect of optical clearing agents on the in vivo optical properties of squamous epithelial tissue. Lasers Surg. Med. 38, 920–927 (2006).
Tuchin, V. V., Bashkatov, A. N., Genina, E. A., Sinichkin, Y. P. & Lakodina, N. A. In vivo investigation of the immersion-liquid-induced human skin clearing dynamics. Tech. Phys. Lett. 27, 489–490 (2001).
Wen, X., Mao, Z. Z., Han, Z. Z., Tuchin, V. V. & Zhu, D. In vivo skin optical clearing by glycerol solutions: mechanism. J. Biophotonics 3, 44–52 (2010).
Zhu, D., Wang, J., Zhi, Z. W., Wen, X. & Luo, Q. M. Imaging dermal blood flow through the intact rat skin with an optical clearing method. J. Biomed. Opt. 15, 026008 (2010).
Zhao, Y. J. et al. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution. Light Sci. Appl. 7, 17153 (2018).
Pires, L. et al. Optical clearing of melanoma in vivo: characterization by diffuse reflectance spectroscopy and optical coherence tomography. J. Biomed. Opt. 21, 081210 (2016).
Zhao, H. et al. A versatile strategy for improving phototherapeutic efficacy on deep-sited tumor by tissue optical clearing technique. Nano Today https://doi.org/10.1016/j.nantod.2020.101058 (2021).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Eng, C. L. et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature 568, 235–239 (2019).
Moffitt, J. R. et al. High-performance multiplexed fluorescence in situ hybridization in culture and tissue with matrix imprinting and clearing. Proc. Natl Acad. Sci. USA 113, 14456–14461 (2016).
Dance, A. Find a home for every imaging data set. Nature 579, 162–163 (2020).
Pleiner, T. et al. Nanobodies: site-specific labeling for super-resolution imaging, rapid epitope-mapping and native protein complex isolation. eLife 4, e11349 (2015).
Schumacher, D., Helma, J., Schneider, A. F. L., Leonhardt, H. & Hackenberger, C. P. R. Nanobodies: chemical functionalization strategies and intracellular applications. Angew. Chem. Int. Ed. 57, 2314–2333 (2018).
Kishi, J. Y. et al. SABER amplifies FISH: enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat. Methods 16, 533–544 (2019).
Shah, S. et al. Single-molecule RNA detection at depth by hybridization chain reaction and tissue hydrogel embedding and clearing. Development 143, 2862–2867 (2016).
Lin, R. et al. A hybridization-chain-reaction-based method for amplifying immunosignals. Nat. Methods 15, 275–278 (2018).
Saritas, T., Puelles, V. G., Su, X.-T., Ellison, D. H. & Kramann, R. Optical clearing and imaging of immunolabeled kidney tissue. J. Vis. Exp. https://doi.org/10.3791/60002 (2019).
Xu, F. et al. High-throughput mapping of a whole rhesus monkey brain at micrometer resolution. Nat. Biotechnol. https://doi.org/10.1038/s41587-021-00986-5 (2021).
Hama, H. et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 14, 1481–1488 (2011).
Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018).
Acknowledgements
The authors thank K. Matsumoto and S. Y. Yoshida for help constructing Figs 5 and 6, respectively, and E. Diel and I. Boothby for preparing samples in Figs 5, 7 and 9. This work was supported by a Japan Science and Technology Corporation (JST) Exploratory Research for Advanced Technology (ERATO) grant (JPMJER2001). H.R.U. was supported by the Science and Technology Platform Program for Advanced Biological Medicine (AMED/MEXT), a Japan Society of the Promotion of Science (JSPS) KAKENHI grant-in-aid for scientific research (JP18H05270), a grant-in-aid from the Human Frontier Science Program and a MEXT Quantum Leap Flagship Program (MEXT QLEAP) grant (JPMXS0120330644). K.M. was supported by a JSPS KAKENHI grant-in-aid for scientific research (20K06885) and a JST Moonshot R&D grant (JPMJMS2023). A.E. was supported by the European Research Council (ERC) Calvaria project, the Vascular Dementia Research Foundation and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy, ID 390857198). K.C. was supported by a Burroughs Wellcome Fund Career Awards at the Scientific Interface, the Searle Scholars Program, the Packard award in Science and Engineering, the NARSAD Young Investigator Award, the McKnight Foundation Technology Award, the JPB Foundation (PIIF and PNDRF), the Institute for Basic Science (IBS-R026-D1) and the NIH grants 1-DP2-ES027992 and U01MH117072. J.W.L. is supported by NIH grants U19NS104653 and P50MH094271. Resources that may help to enable general users to establish the methodology are freely available online at http://www.chunglabresources.org.
Author information
Authors and Affiliations
Contributions
Introduction (D.S.R. and J.W.L.); Experimentation (D.S.R., W.G., K.M., C.P., K.C., A.E., H.R.U. and J.W.L.); Results (D.S.R., W.G., K.M., C.P., K.C., A.E., H.R.U. and J.W.L.); Applications (D.S.R., W.G., K.M., C.P., K.C., A.E., H.R.U. and J.W.L.); Reproducibility and data deposition (D.S.R., W.G., K.M., C.P., K.C., A.E., H.R.U. and J.W.L.); Limitations and optimizations (D.S.R., W.G., K.M., C.P., K.C., A.E., H.R.U. and J.W.L.); Outlook (D.S.R. and J.W.L.); overview of the Primer (D.S.R. and J.W.L.).
Corresponding author
Ethics declarations
Competing interests
H.R.U. is co-founder of CUBICStars, Inc. and a co-inventor on the following patent applications covering the CUBIC reagents: PCT/JP2014/070618 (pending, patent applicant is RIKEN, other co-inventors are E. A. Susaki and K. Tainaka); PCT/JP2017/016410 (pending, patent applicant is RIKEN, other co-inventors are K. Tainaka and T. Murakami). K.C. is an inventor for patent applications covering some technologies described in this paper and co-founder of LifeCanvas Technologies. A.E. and C.P. have filed a patent on whole-body clearing and imaging related technologies. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Methods Primers thanks Alan King Lun Liu, Woong Sun, Valery Tuchin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Aivia: https://www.aivia-software.com/
Arivis Vision4D: https://imaging.arivis.com/en/imaging-science/arivis-vision4d
Cell Image Library: http://www.cellimagelibrary.org/home
ilastik: https://www.ilastik.org/
Image Data Resource: http://idr.openmicroscopy.org/about/
Imaris: https://imaris.oxinst.com/
Napari: https://napari.org/
Online repository for tissue clearing-validated antibodies: https://idisco.info/validated-antibodies/
Trainable Weka Segmentation: https://imagej.net/plugins/tws/
Glossary
- Autofluorescence
-
Fluorescence that arises from endogenous fluorescent molecules contained within a biological specimen. Can also be introduced exogenously (that is, some hydrogels autofluoresce).
- Dipping objectives
-
Objective lenses that are designed to be submerged into a liquid. Dipping objectives are found on upright microscopes, and samples are mounted without a coverslip.
- Lattice light sheets
-
Light sheets that are formed using a specialized interference pattern that results in the projection of thin beams of excitation light into a sample, which are rapidly dithered to form the light sheet.
- Steric hindrances
-
Refers to the inability to mount a sample on a microscope when the working distance of the objective is shorter than the thinnest dimension of the sample.
- Optical section
-
An image of a 2D plane within a 3D object that is derived by optical, rather than mechanical, means.
- Isotropic spatial resolution
-
Refers to specialized light microscopy techniques that produce an identical lateral and axial resolution.
- Network switch
-
A computer network hardware device that allows multiple computers to communicate.
- Dispersion
-
A measure of the change in refractive index relative to the wavelength of light passing through a substance. If a substance has high dispersion, it means blue light and red light will refract differently when passing through it.
- Random forests
-
Machine learning algorithms that comprise many ‘estimators’ that each make a prediction as to which segmentation group a pixel should belong. When many estimators are combined into a ‘forest’, the final prediction is highly accurate.
- Tortuosity
-
Describes the degree of curvature and/or twist in a blood vessel.
Rights and permissions
About this article
Cite this article
Richardson, D.S., Guan, W., Matsumoto, K. et al. Tissue clearing. Nat Rev Methods Primers 1, 84 (2021). https://doi.org/10.1038/s43586-021-00080-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-021-00080-9
This article is cited by
-
Whole-body cellular mapping in mouse using standard IgG antibodies
Nature Biotechnology (2024)
-
Signal improved ultra-fast light-sheet microscope for large tissue imaging
Communications Engineering (2024)
-
Reflective multi-immersion microscope objectives inspired by the Schmidt telescope
Nature Biotechnology (2024)
-
Imaging the biological microcosmos with a tiny telescope
Nature Biotechnology (2024)
-
Spatial analysis of tissue immunity and vascularity by light sheet fluorescence microscopy
Nature Protocols (2024)