Abstract
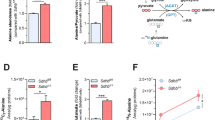
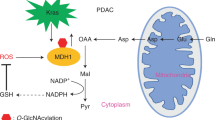
The oncogenic KRAS mutation has a critical role in the initiation of human pancreatic ductal adenocarcinoma (PDAC) since it rewires glutamine metabolism to increase reduced nicotinamide adenine dinucleotide phosphate (NADPH) production, balancing cellular redox homeostasis with macromolecular synthesis1,2. Mitochondrial glutamine-derived aspartate must be transported into the cytosol to generate metabolic precursors for NADPH production2. The mitochondrial transporter responsible for this aspartate efflux has remained elusive. Here, we show that mitochondrial uncoupling protein 2 (UCP2) catalyses this transport and promotes tumour growth. UCP2-silenced KRASmut cell lines display decreased glutaminolysis, lower NADPH/NADP+ and glutathione/glutathione disulfide ratios and higher reactive oxygen species levels compared to wild-type counterparts. UCP2 silencing reduces glutaminolysis also in KRASWT PDAC cells but does not affect their redox homeostasis or proliferation rates. In vitro and in vivo, UCP2 silencing strongly suppresses KRASmut PDAC cell growth. Collectively, these results demonstrate that UCP2 plays a vital role in PDAC, since its aspartate transport activity connects the mitochondrial and cytosolic reactions necessary for KRASmut rewired glutamine metabolism2, and thus it should be considered a key metabolic target for the treatment of this refractory tumour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data used for the expression analysis and GSEA are publicly available. The datasets using HG133plus2 from Affymetrix included ArrayExpress accession no. E-MEXP-2780 and Gene Expression Omnibus accession nos. GSE15471, GSE22780, GSE16515, GSE32676, GSE42952 and GSE36133. The dataset using HG_U219 from Affymetrix included ArrayExpress accession no. E-MTAB-6134. The unverified heterozygous mutation (c.34G>C, p.G12R) for KP-2 cells is reported in the COSMIC with sample ID COSS1298218. There are no restrictions on data availability. Any additional information supporting the data associated with this paper and the findings of this study is available from the corresponding authors upon request. Source data are provided with this paper.
References
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Hensley, C. T., Wasti, A. T. & DeBerardinis, R. J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Abrego, J. et al. GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells. Cancer Lett. 400, 37–46 (2017).
Chakrabarti, G. et al. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by β-lapachone. Cancer Metab. 3, 12 (2015).
Wang, Y.-P. et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol. Cell 64, 673–687 (2016).
Vozza, A. et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl Acad. Sci. USA 111, 960–965 (2014).
Pecqueur, C. et al. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J. Biol. Chem. 276, 8705–8712 (2001).
Rupprecht, A., Moldzio, R., Mödl, B. & Pohl, E. E. Glutamine regulates mitochondrial uncoupling protein 2 to promote glutaminolysis in neuroblastoma cells. Biochim. Biophys. Acta, Bioenerg. 1860, 391–401 (2019).
Nübel, T. et al. Modified glutamine catabolism in macrophages of Ucp2 knock-out mice. Biochim. Biophys. Acta 1777, 48–54 (2008).
Donadelli, M., Dando, I., Fiorini, C. & Palmieri, M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 71, 1171–1190 (2014).
Bouillaud, F., Couplan, E., Pecqueur, C. & Ricquier, D. Homologues of the uncoupling protein from brown adipose tissue (UCP1): UCP2, UCP3, BMCP1 and UCP4. Biochim. Biophys. Acta 1504, 107–119 (2001).
Palmieri, L. et al. Citrin and aralar1 are Ca2+‐stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 20, 5060–5069 (2001).
Owen, O. E., Kalhan, S. C. & Hanson, R. W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 277, 30409–30412 (2002).
Alkan, H. F. et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 28, 706–720.e6 (2018).
Yoo, H. C. et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 31, 267–283.e12 (2020).
Dalla Pozza, E. et al. Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim. Biophys. Acta 1823, 1856–1863 (2012).
Lee, W. D., Mukha, D., Aizenshtein, E. & Shlomi, T. Spatial-fluxomics provides a subcellular-compartmentalized view of reductive glutamine metabolism in cancer cells. Nat. Commun. 10, 1351 (2019).
Cavero, S. et al. Identification and metabolic role of the mitochondrial aspartate-glutamate transporter in Saccharomyces cerevisiae. Mol. Microbiol. 50, 1257–1269 (2003).
Porcelli, V. et al. Molecular identification and functional characterization of a novel glutamate transporter in yeast and plant mitochondria. Biochim. Biophys. Acta Bioenerg. 1859, 1249–1258 (2018).
Palmieri, F. Mitochondrial transporters of the SLC25 family and associated diseases: a review. J. Inherit. Metab. Dis. 37, 565–575 (2014).
Monné, M., Miniero, D. V., Iacobazzi, V., Bisaccia, F. & Fiermonte, G. The mitochondrial oxoglutarate carrier: from identification to mechanism. J. Bioenerg. Biomembr. 45, 1–13 (2013).
Tessa, A. et al. Identification of novel mutations in the SLC25A15 gene in hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome: a clinical, molecular, and functional study. Hum. Mutat. 30, 741–748 (2009).
Fiermonte, G. et al. An adult with type 2 citrullinemia presenting in Europe. N. Engl. J. Med. 358, 1408–1409 (2008).
Palmieri, L. et al. Identification in Saccharomyces cerevisiae of two isoforms of a novel mitochondrial transporter for 2-oxoadipate and 2-oxoglutarate. J. Biol. Chem. 276, 1916–1922 (2001).
Palmieri, F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 447, 689–709 (2004).
Cassago, A. et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc. Natl Acad. Sci. USA 109, 1092–1097 (2012).
Baffy, G. Uncoupling protein-2 and cancer. Mitochondrion 10, 243–252 (2010).
Gao, J. et al. Validation of targeted next-generation sequencing for RAS mutation detection in FFPE colorectal cancer tissues: comparison with Sanger sequencing and ARMS-Scorpion real-time PCR. BMJ Open 6, e009532 (2016).
Rudin, C. M. et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 44, 1111–1116 (2012).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Wiederschain, D. et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle 8, 498–504 (2009).
Barger, C. J., Branick, C. & Chee, L. & Karpf, A. R. Pan-cancer analyses reveal genomic features of FOXM1 overexpression in cancer. Cancers (Basel) 11, 251 (2019).
Lou, E. et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 7, e33093 (2012).
Wach, A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12, 259–265 (1996).
Agrimi, G., Russo, A., Scarcia, P. & Palmieri, F. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem. J. 443, 241–247 (2012).
Bustin, S. A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25, 169–193 (2000).
Kushnirov, V. V. Rapid and reliable protein extraction from yeast. Yeast 16, 857–860 (2000).
De Marcos Lousa, C., Trézéguet, V., Dianoux, A.-C., Brandolin, G. & Lauquin, G. J. The human mitochondrial ADP/ATP carriers: kinetic properties and biogenesis of wild-type and mutant proteins in the yeast S. cerevisiae. Biochemistry 41, 14412–14420 (2002).
Li, Y. et al. Functional characterization of the partially purified Sac1p independent adenine nucleotide transport system (ANTS) from yeast endoplasmic reticulum. J. Biochem. 164, 313–322 (2018).
Vozza, A. et al. Biochemical characterization of a new mitochondrial transporter of dephosphocoenzyme A in Drosophila melanogaster. Biochim. Biophys. Acta Bioenerg. 1858, 137–146 (2017).
Badea, L., Herlea, V., Dima, S. O., Dumitrascu, T. & Popescu, I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology 55, 2016–2027 (2008).
Pei, H. et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 16, 259–266 (2009).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Donahue, T. R. et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin. Cancer Res. 18, 1352–1363 (2012).
Van den Broeck, A., Vankelecom, H., Van Eijsden, R., Govaere, O. & Topal, B. Molecular markers associated with outcome and metastasis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 31, 68 (2012).
Winter, C. et al. Google goes cancer: improving outcome prediction for cancer patients by network-based ranking of marker genes. PLoS Comput. Biol. 8, e1002511 (2012).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Puleo, F. et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology 155, 1999–2013.e3 (2018).
Marchi, S., Bonora, M., Patergnani, S., Giorgi, C. & Pinton, P. Methods to assess mitochondrial morphology in mammalian cells mounting autophagic or mitophagic responses. Methods Enzymol. 588, 171–186 (2017).
Divakaruni, A. S. et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl Acad. Sci. USA 110, 5422–5427 (2013).
Young, J. D., Allen, D. K. & Morgan, J. A. Isotopomer measurement techniques in metabolic flux analysis II: mass spectrometry. Methods Mol. Biol. 1083, 85–108 (2014).
Acknowledgements
This work was supported by a grant from the Italian Association for Cancer Research (AIRC no. IG 2014 Id.15404 to G.F.) and a grant from the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (no. 2017PAB8EM_002 to G.F.).
Author information
Authors and Affiliations
Contributions
S.R., C.P., A.V., D.F. and L.C. performed most of the in vitro experiments. F.D. performed the in vitro reconstitution experiments. I.P. performed the cytofluorimetric analyses. S.N.B. and F.M.L. performed the respirometry experiments. R.G., G.E.D. and G.A. performed the GS–MS metabolomics experiments. P.S. carried out the RT–PCR experiments. Y.L., C.M.T.M. and F.P. performed the yeast experiments. V.R., R.M. and V.D. performed the xenograft experiments. M.R.G., R.A.C. and S.J.R. performed the organotypic cell culture experiments. M.C.V. performed the GSEA and analysis on GEP. L.P., V.D. and G.F. conceived and designed the study. W.S., L.P., V.D., E.M., C.L.R. and G.F. interpreted the results and wrote the manuscript. G.F. acquired the funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editors: Elena Bellafante; Christoph Schmitt. Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Expression analysis of UCP2 and UCP2 homologs in PDAC cells.
a, Transcript abundance of UCP2 in PDAC primary tumour samples and normal pancreatic tissues derived from gene expression profiling (GEP) of different publicly available datasets. b–d, The relative expression of UCP2 in control and UCP2shRNA-transfected PDAC cells. UCP2 transcript levels were quantified by qPCR (b and d) and UCP2 protein levels were assayed with a UCP2-specific antiserum (c). An anti-COX4 antibody was used for normalization. e, f, UCP2 silencing does not alter the expression levels of other UCP homologs. The relative expression of all UCP genes was quantified by qPCR. The ΔCT of the indicated UCP homolog gene (e) and that of UCP2 (f) of the CtrshRNA-transfected cells were used as internal calibrators. g, ATP/ADP and Pi/Asp exchanges activities catalysed by mitochondrial extracts of control, UCP2-silenced and UCP2-silenced rescued PDAC cells. Mitochondria were solubilized with detergent and reconstituted into liposomes containing Pi or ATP. The exchange reaction was started by adding [14C]aspartate or [14C]ADP to proteoliposomes and stopped after 30 minutes with inhibitors. Values represent means ± SD of three independent experiments. h, Expression of murine Ucp2 (mUcp2) gene in Patu8988T cells. The relative expression of human UCP2 and murine Ucp2 were determined by Sybr green qPCR. The ΔCT of human UCP2 of the CtrshRNA-transfected cells carrying the empty expression vector (EV) was used as internal calibrator. The protein expression levels of panel h are shown in panel c. i, Effect of glutamine on UCP2 protein expression levels. The total cell extracts were assayed in the presence or absence (overnight starvation) of glutamine. For all transcript analyses, reverse transcribed cDNA from three biological replicates was used and three technical replicates were analysed for each biological replicate, values represent means ± SD (b, d–f, h). COX4 normalization was carried out on the same SDS-PAGE used to assay UCP2 expression levels, similar results were obtained in three biologically independent experiments (c, i). Statistical significance was calculated by unpaired two-tailed Mann–Whitney U-test (a). rUCP2, recombinant human UCP2. Patu8988TNI, cells transfected with non-inducible lentiviral plasmids.
Extended Data Fig. 2 UCP2 silencing reduces the availability of cytosolic aspartate in PDAC cell lines.
a–f, UCP2 silencing reduces the clonogenic capacity of Patu8988T and Panc1 but not those of BxPC3 and KP-2 cells. The clonogenic assays corresponding to Fig. 1f are shown (a–d). Aspartate in the medium rescues the clonogenic defect of UCP2-silenced KRASmut PDAC cells (a, b and e, f). The histogram data were referred to dox-induced CtrshRNA-transfected cells without aspartate. g, The presence of glutamate (1 mM) in the medium partially rescues the proliferation defect induced by UCP2 silencing in KRASmut PDAC cells. Doxycycline was always present in e–g. h, UCP2 silencing does not alter the expression of the mitochondrial aspartate/glutamate carrier (AGC) in PDAC cells, similar results were obtained in three biologically independent experiments. COX4 normalization was carried out on the same SDS-PAGE used to assay AGC1/2 expression levels (h). i, An oversimplification of aspartate export out of mitochondria catalysed by UCP2 or AGC in the presence or limited availability of glutamine, respectively. Values represent means ± SD of two biologically independent triplicates. Statistical significance was calculated by unpaired two-tailed Student’s t-test.
Extended Data Fig. 3 UCP2 has key role in redox homeostasis of PDAC cells.
a, GSH or NAC in the medium fully rescues the growth defect of UCP2-silenced Panc1 cells. b, UCP2 silencing reduces the GSH/GSSG ratio only in KRASmut cells. c–i, UCP2 silencing increases ROS levels only in KRASmut cells. The cytofluorimetric raw data of ROS analysis in control and UCP-silenced cells are shown (d–i). j, UCP2 silencing reduces the NADPH/NADP+ ratio only in KRASMUT cells. The histogram data were referred to CtrshRNA-transfected cells (b, c and j). k, l, In PDAC, UCP2 expression levels are associated to those of genes involved in the redox homeostasis. The GSEA panels show the enrichment of gene sets related to UCP2 expression in roughly 300 PDAC primary tumour samples from E-MTAB-6134 dataset (k) and 43 PDAC cell lines from dataset GSE36133 (l). NES, normalized enrichment score. m, Fluorescence microscopy images of mitochondrial-targeted GFP transfected Patu8988T and BxPC3 cells before and after 4 minutes of incubation with digitonin fractionation buffer. Scale bar = 10 µm. Values represent means ± SD of two biologically independent triplicates. Statistical significance was calculated by unpaired two-tailed Student’s t-test; Welch correction was applied to shRNA1 vs shRNA1/GSH (a), Ctr vs shRNA1 and Ctr vs shRNA2 (Panc1) (c).
Extended Data Fig. 4 In yeast cells, UCP2 catalyses the export of aspartate out of mitochondria in exchange for phosphate.
a, The yeast mitochondrial aspartate/glutamate (AGC1p) and the two isoforms of oxoadipate carrier (ODC1p/ODC2p), which exchange 2-ketoglutarate against malate, are the mitochondrial carriers involved in the yeast malate-aspartate shuttle (MAS) required for the NADH oxidation derived from the peroxisomal oxidation of oleate. b, The UCP2 aspartate/Pi exchange activity together with the glutamate + H+ symport catalysed by the yeast YMC2p restores the loss of AGC1p in MAS. c, The bacterial expressed UCP2R279Q mutant does not catalyse any aspartate/Pi exchange activity once reconstituted into liposomes. Values represent means ± SD of three independent experiments. d, A representative immunoblot of expression of UCP2 and UCP2R279Q V5-tagged proteins in W303 yeast cells used in panel e, similar results were obtained in three biologically independent experiments and in the yeast models reported in Fig. 2i–k. A yeast anti-porin antiserum was used for protein normalization. Porin normalization was carried out on the same SDS-PAGE used to assay V5-tagged UCP2 expression levels (d). e, Serial dilutions of different yeast cell models expressing UCP2 or UCP2R279Q were spotted on YP plate in the presence of oleate. f, To rescue the growth defect of the ΔAGC1 yeast strain on oleate, UCP2 requires the presence of the mitochondrial isoform of the aspartate transaminase (AAT1). g, h, In the inner mitochondrial membrane UCP2 catalyses only the aspartate/Pi exchange reaction. The two models show other two possible exchange activities catalysed by the recombinant UCP2 into liposomes, aspartate/malate and malate/oxaloacetate, which were not confirmed in the yeast models used. Asp, aspartate; Glu, glutamate; Mal, malate; α-KG, α-ketoglutarate; OA, oxaloacetate, MDH, malate dehydrogenase; GDH glutamate dehydrogenase.
Extended Data Fig. 5 UCP2 silencing inhibits KRASmut PDAC cells growth in organotypic 3D and xenograft models.
a–b, UCP2 silencing reduces growth of organotypic cultures of KRASmut PDAC cells. Representative microscopic images of growth and death (red signal) of PDAC cells grown on 100% Matrigel ECM in the presence of doxycycline. Scale bar = 500 µm. Histograms show the growth and the death of cells cultured for seven days. The growth data were normalized to those measured at the beginning of the experiment (t0), whereas the death data were referred to the CtrshRNA-transfected cells. c-f, UCP2 silencing inhibits the proliferation of KRASmut PDAC cells in vivo. The Patu8988T and BxPC3 tumour volumes reported in Fig. 3, panels c and d are shown (c, e). g, h, Representative images of tumour sizes and Ki67 immunostaining, taken at the end point, generated by Panc1 and KP-2 cells injected in female SCID mice (n = 5). Inset shows localization at higher magnification (40X), scale bar=25 μm (g, h). i, Tumour weights of all xenograft experiments. j, Percentage of Ki67 positive PDAC cells. k, l, UCP2 silencing reduces the GSH/GSSG and NADPH/NADP+ ratios only in KRASmut cell xenografts, the histogram data were referred to CtrshRNA xenografts. Values represent means ± SD of two biologically independent triplicates (a and b) and five mice (c-f and i-l). Statistical significance was calculated by unpaired two-tailed Student’s t-test (a-f, i, j and l) and unpaired two-tailed Mann–Whitney U-test (k). RFU, relative fluorescence unit.
Extended Data Fig. 6 Murine Ucp2 rescues the growth defect of UCP2-silenced KRASmut PDAC cells.
a, mUcp2 expression rescues the clonogenic defect of UCP2-silenced KRASmut PDAC cells. b, Cytofluorimetric raw data of ROS analysis carried out in the rescue experiment reported in Fig. 4, panel b. c, d, UCP2 silencing reduces the GSH/GSSG and NADPH/NADP+ ratios in Patu8988TNI also in the absence of doxycycline, the histogram data were referred to the CtrshRNA-transfected cells. e, UCP2 silencing reduces oxygen consumption rates (OCR) in the presence of glutamine in Patu8988TNI also in the absence of doxycycline, values represent means ± SD of three biologically independent experiments. f, mUcp2 expression recovers the altered cytosol/matrix distribution of the glutamine-derived metabolites of UCP2-silenced PDAC cells. g-i, Tumour volumes, tumour weights and the percentage of Ki67 positive cells of Patu8988T tumours reported in Fig. 4e are shown (n = 5). Asp, aspartate; Glu, glutamate; Mal, malate; 2-KG, 2-ketoglutarate. Patu8988TNI, cells transfected with non-inducible lentiviral plasmids. Values represent means ± SD of two biologically independent triplicates (a, c, d and f) and five mice (g-i). Statistical significance was calculated by unpaired two-tailed Student’s t-test. Welch correction was applied to Mito_CtrNI vs Mito_shRNA1NI (Asp), Mito_CtrNI vs Mito_shRNA1NI (Glu), Mito_shRNA1NI vs Mito_shRNA1NI(rescued) (Glu) and Mito_shRNA1NI vs Mito_shRNA1NI(rescued) (Mal) (f).
Extended Data Fig. 7 Expression of KRASG12V in BxPC3 cells does not alter the expression levels of UCP2.
a, Expression of HA-tagged KRASG12V in control and UCP2shRNA-transfected BxPC3 cells. b, c The relative expression of UCP2 in control and UCP2shRNA-transfected BxPC3 cells expressing or not expressing KRASG12V. UCP2 transcript levels were quantified by qPCR (b) and UCP2 protein levels were assayed with a UCP2-specific antiserum (c). An anti-COX4 antibody was used for norKP-2malization. COX4 normalization was carried out on the same SDS-PAGEs used to assay HA-tagged KRASG12V (a) and UCP2 (c) expression levels. Similar results were obtained in three biologically independent experiments (a and c). For transcript analyses, reverse transcribed cDNA from three biological replicates was used and three technical replicates were analysed for each biological replicate, values represent means ± SD of three independent experiments (b). BxPC3NI, cells transfected with non-inducible lentiviral plasmids. rUCP2, recombinant human UCP2.
Extended Data Fig. 8 KRAS allele imbalance in KP-2 PDAC cell lines.
Two examples of forward and reverse sequences of PCR KRAS fragments (amplified from a first-strand cDNA) carried out along the study. The dotted line indicates difference in the pick’s area between the wild-type sequence and the unverified heterozygous mutation (c.34G>C, p.G12R) found in the COSMIC database. The GGT codon encoding for the wild-type glycine 12 is underlined.
Extended Data Fig. 9 A schematic representation of lentiviral plasmids used to verify the dependency of mutated KRAS on UCP2 in BxPC3 cells.
a, b, The scramble and UCP2 silencing (shRNA1) non-inducible pLKO.1 (Blast) plasmids, used as controls, showing the BamHI and KpnI restriction sites used to clone the KRASG12V expression cassette. c, d, The scramble and UCP2 silencing (shRNA1) non-inducible pLKO.1 (Blast) plasmids carrying the KRASG12V expression cassette downstream the blasticidin resistance gene (BSD). HA-tagged KRASG12V was cloned under the control of the UbC promoter and in frame with EGFP and a "self-cleaving" P2A peptide. The efficiency of transfection (about 85%) was measured by following the EGFP fluorescence whereas the presence of P2A allowed the release of the HA-tagged KRASG12V.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 2
Supplementary Table 1
Mean expression of genes corresponding to GSEA gene set in PDAC primary cases and PDAC cell lines
Source data
Source Data Fig. 1
Statistical Source Data of Fig. 1
Source Data Fig. 2
Statistical Source Data of Fig. 2
Source Data Fig. 3
Statistical Source Data of Fig. 3
Source Data Fig. 4
Statistical Source Data of Fig. 4
Source Data Extended Data Fig. 1
Statistical Source Data of Extended Data Fig. 1
Source Data Extended Data Fig. 1
Unprocessed western blot
Source Data Extended Data Fig. 2
Statistical Source Data of Extended Data Fig. 2
Source Data Extended Data Fig. 2
Unprocessed western blot
Source Data Extended Data Fig. 3
Statistical Source Data of Extended Data Fig. 3
Source Data Extended Data Fig. 4
Unprocessed western blot
Source Data Extended Data Fig. 5
Statistical Source Data of Extended Data Fig. 5
Source Data Extended Data Fig. 6
Statistical Source Data of Extended Data Fig. 6
Source Data Extended Data Fig. 7
Unprocessed western blot
Rights and permissions
About this article
Cite this article
Raho, S., Capobianco, L., Malivindi, R. et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metab 2, 1373–1381 (2020). https://doi.org/10.1038/s42255-020-00315-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00315-1
This article is cited by
-
Metabolic difference between patient-derived xenograft model of pancreatic ductal adenocarcinoma and corresponding primary tumor
BMC Cancer (2024)
-
Glutamine addiction in tumor cell: oncogene regulation and clinical treatment
Cell Communication and Signaling (2024)
-
UCP2 and pancreatic cancer: conscious uncoupling for therapeutic effect
Cancer and Metastasis Reviews (2024)
-
Mitochondrial dysfunction at the crossroad of cardiovascular diseases and cancer
Journal of Translational Medicine (2023)
-
SLC25A32 promotes malignant progression of glioblastoma by activating PI3K-AKT signaling pathway
BMC Cancer (2023)