Abstract
Whether and how the reactive oxygen species generated by hepatic stellate cells (HSCs) promote immune evasion of hepatocellular carcinoma (HCC) remains mysterious. Therefore, investigating the function of superoxide anion (O2•−), the firstly generated reactive oxygen species, during the immune evasion become necessary. In this work, we establish a novel in situ imaging method for visualization of O2•− changes in HSCs based on a new two-photon fluorescence probe TPH. TPH comprises recognition group for O2•− and HSCs targeting peptides. We observe that O2•− in HSCs gradually rose, impairing the infiltration of CD8+ T cells in HCC mice. Further studies reveal that the cyclin-dependent kinase 4 is deactivated by O2•−, and then cause the up-regulation of PD-L1. Our work provides molecular insights into HSC-mediated immune evasion of HCC, which may represent potential targets for HCC immunotherapy.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death, posing a serious threat to people’s health and lives1,2. In recent decades, a variety of immunotherapy strategies have been developed for the treatment of HCC3,4. Although have shown great promises, their clinical therapeutic efficacy was reduced by the immune evasion3. Immune evasion refers to the process through which cancer cells inhibit or decrease the body’s immune response to malignancy. Undoubtedly, immune evasion is regarded as an indispensable strategy for HCC cell survival5,6. Immune evasion in HCC complicates the treatment, and also increases the risk of tumor metastasis and postoperative recurrence. Therefore, understanding the molecular mechanism of immune evasion can provide vital insight for HCC treatment.
Hepatic stellate cells (HSCs) account for 10% of the nonparenchymal cells in the liver. HSCs are crucial mediators in regulating the physiological and pathological processes of liver7. When stimulated by injuries or immune responses, the HSCs in quiescent state transform into active state (aHSCs)8. Subsequently, aHSCs regulate liver fibrosis and immune processes via secreting active molecules such as the extracellular matri, cytokines, and reactive oxygen species (ROS) through lipid metabolism9,10. Current studies have shown that abnormal aHSCs could cause the immune evasion of HCC cells11,12,13. Coulouarn et al. found that HCC cells upregulated the expression of vascular endothelial growth factor A in HSCs, causing the promotion of angiogenesis at tumor sites12. In addition, aHSCs promoted migration and invasion of HCC cells through focal adhesion kinase-matrix metalloproteinase 9 signaling13. However, the detailed molecular mechanisms still need to be explored.
Superoxide anion (O2•−), as the first ROS produced in organisms, profoundly affects the immune responses of HCC14,15. The activation of HSCs is accompanied by lipid metabolism and oxidative phosphorylation, providing energy to maintain the normal physiological functions of the cells. Importantly, oxidative phosphorylation is an important pathway to produce O2•−. Therefore, we assume that during this process, the levels of O2•− may change distinctly. However, due to the high activity and short duration of O2•−, no suitable method for real-time and in situ detecting O2•− in HSCs has been reported. Thus, whether and how O2•− produced in aHSCs regulate immune evasion of HCC remains mysterious. Therefore, it is urgent to develop a tool that can be used to explore the flux of O2•− in HSCs, and investigate the detailed mechanism of HCC immune evasion mediated by O2•−.
Currently, two-photon fluorescence imaging technique with high sensitivity and real-time detection has served as a superior approach to monitor molecule events in vitro and in vivo16,17,18. This technique has excellent repeatability, and can greatly avoid the interference of biological tissue absorption by its two-photon absorption property. With the help of two-photon fluorescence imaging technique, our group had observed the dynamic, reversible changes of O2•− in the liver injury mediated by ischemia-reperfusion and in brains of mice with depression17,18. However, there is still a lack of imaging material for two-photon fluorescence imaging of O2•− level changes in HSCs.
To solve the above problems, we designed a two-photon fluorescence probe TPH for imaging O2•− in HSCs. TPH was composed of CGPTAKYIC19 for HSCs-specific targeting and caffeic acid residue as a recognition group for specific detection of O2•−. In the presence of O2•−, caffeic acid residues undergo phenol–quinone tautomerization, resulting in enhanced fluorescence (Fig. 1). Using TPH, we investigated the function of O2•− during the activation of HSCs in vitro and in vivo. Further research found that excess O2•− caused dysfunction of cyclin-dependent kinase 4 (CDK4), which in turn led to increased programmed cell death-ligand 1 (PD-L1) levels. Ultimately, these changes promoted the immune evasion of HCC.
Results and discussion
Optical properties of TPH
The synthesis and structural characterizations of TPH were shown in Supplementary Figs. 1–3. Prior to the in vivo application, we investigated the photophysical properties of TPH. As shown in Fig. 2a, the absorption maximum peak of TPH was 320 nm, which would shift to 370 nm after reacting with O2•−. Next, the fluorescence response of TPH to O2•− was tested. The addition of O2•− resulted in an increased fluorescence intensity due to the oxidation of the phenolic hydroxyl group in TPH by O2•− (Fig. 2b). The fluorescence intensities of TPH at 490 nm increased gradually as O2•− was added in the range of 0–20 μM. The linear equation was F = 245.32 [O2•−] (μM) + 329.11, with a linear correlation coefficient of 0.992. The limit of detection (LOD) was 111 nM, as calculated by the equation LOD = 3σ/K (Fig. 2c, d). To interrogate the specificity of TPH toward O2•−, we examined the fluorescence responses of TPH to O2•− and other common interfering substances under simulated physiological conditions. The probe can also recognize O2•− under two-photon excitation of 800 nm (Supplementary Fig. 4). Encouragingly, only O2•− resulted in a significant increase of the fluorescence signal at 490 nm without any interference from other highly active molecules in vivo (Supplementary Fig. 5). This suggested that TPH showed excellent specificity to O2•−. Moreover, TPH exhibits the reversible response to O2•−. In addition, TPH possessed other advantages including instantaneous response, good photostability and favorable biocompatibility (Supplementary Figs. 6–12). The above results highlight that the probe could be a promising candidate for O2•− detection during the activation of HSCs.
O2 •− detection during the activation of HSCs
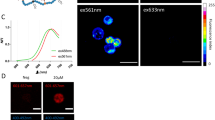
Motivated by the preeminent photophysical properties of TPH, we explored its capability of imaging O2•− during the activation of HSCs. Cell selectivity experiments confirmed that TPH could specifically target HSCs (Supplementary Fig. 13). First, we verified whether the TPH could recognize O2•− in HSCs. LX-2 cells were pretreated with 2-methoxyestradiol (2-Me) to boost the endogenous O2•− level20,21. As shown in Fig. 3a, b, compared with the control group, 2-Me treated cells elicited brighter fluorescence. In contrast, the fluorescence intensity was significantly reduced when Tiron (O2•− scavenger) was added to those cells22. These images illustrated that O2•− in HSCs could be tested by TPH. Subsequently, we explored the changes of O2•− levels during the activation of HSCs. We successfully activated hepatic stellate cells using TGF-β1 (Supplementary Fig. 14)23. As displayed in Fig. 3c, d, cells with TGF-β1 incubation showed significantly increased fluorescence compared with the control group. Moreover, the fluorescence intensity of the Tiron group was lower than that of the TGF-β1 group. Moreover, the imaging results of O2•− level changes during HSCs activation could be stimulated performed under both one-photon and two-photon excitations (Supplementary Fig. 15).
a O2•− fluorescence imaging of endogenous O2•− in LX-2 cells. a1: control; a2: 2-Me (0.1 µg mL−1) for 15 min; a3: after 2-Me incubation, Tiron (10 µM) was added and incubated for 30 min. c O2•− fluorescence imaging of LX-2 cell activation. c1: control; c2: TGF-β1 (5 ng mL−1) was incubated for 12 h; c3: TGF-β1 was incubated and Tiron (10 µM) was added for 30 min. b, d Fluorescence intensities of (a) and (c). Scale bar = 50 μm (a) or 10 μm (c). The data were expressed as mean ± SD, n = 3. *p < 0.01, **p < 0.05, ***p < 0.001 compared to the control group.
The finding indicated that a large amount of O2•− was produced during the activation of HSCs. Overall, we successfully established an imaging method for detecting O2•− within HSCs.
The activation degrees of HSCs and the levels of intracellular O2 •−
Next, the relationship between the activation degrees of HSCs and the O2•− levels was explored by the established imaging method. To build the HSCs with varying activation degrees, LX-2 cells were pretreated with different stimulation conditions. As illustrated in Fig. 4, with the increasing of concentration or incubation time, the stronger fluorescence was observed. The above data revealed that the activation degree of HSCs was positively correlated with O2•− level.
O2 •− imaging in mouse HSCs with different activation degrees
Encouraged by the results of the cell experiments, we continued to explore the potential of TPH applications in vivo. The mice with various levels of aHSCs were obtained by intraperitoneal injection carbon tetrachloride (CCl4) solution (Supplementary Fig. 16)10,24. Next, TPH was injected intraperitoneally into the mice before confocal microscope imaging. Compared with the control groups, brighter fluorescence signals were observed in mice with more aHSCs (Fig. 5a–c). These meant that the levels of O2•− were increased in mice with aHSCs. Of note, the fluorescence intensities were enhanced 3.0-fold (2 weeks), 4.7-fold (4 weeks), and 10.6-fold (6 weeks), respectively. These findings revealed that as the activation degree of mouse HSCs increased, the O2•− levels in HSCs enhanced. Finally, D-penicillamine (D-pen) and tocopherol (VE) were used to inhibit the activation of HSCs25,26. A weaker fluorescence was observed in mice with D-pen or VE treated than in mice with CCl4 only (Fig. 5d and Supplementary Fig. 17). This result suggested that inhibiting the activation of mouse HSCs could reduce the concentration of O2•−. Collectively, all these data implied that TPH could be used for the detection of O2•− in mouse HSCs. With the help of TPH, we find that aHSCs produced the excessive O2•− in mice.
a–c Mice were intraperitoneally injected with olive oil or CCl4 solution for 2, 4, and 6 weeks, respectively. Control: olive oil; CCl4: CCl4 solution. d Fluorescence imaging of mice with inhibition of HSCs activation. The mice were first given CCl4 solution for 6 weeks, followed by 2 weeks of either stroke-physiological saline solution or D-pen. The data were expressed as mean ± SD, n = 3. ***p < 0.001 compared to the control group.
The O2 •− level and the immunosuppressive ability of HCC microenvironment
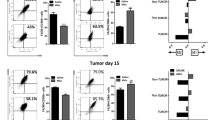
Researches established a closely link between O2•− and the immunosuppressive microenvironment of HCC15,27. The anti-tumor activity of CD8+ T cells inside HCC is affected by ROS28,29,30. Considering the excessive O2•− in the aHSCs of mice, we investigated the relationship between O2•− in aHSCs and the activity of CD8+ T cells in HCC mice. We constructed orthotopic mouse models of HCC containing quiescent-HSCs or aHSCs, and performed fluorescence imaging and immunofluorescence imaging. As shown in Fig. 6a, c, the fluorescence intensities of aHSCs–HCC mice were significantly higher than that of control HCC mice. On the contrary, the proportions of CD8+ T cells around the tumor were decreased in aHSCs–HCC mice. This suggests a negative correlation between the two biological events: inhibition of HSCs activation decreased the level of O2•− and increased the proportion of CD8+ T cells around the tumor (Fig. 6b, d and Supplementary Figs. 18–20). These results attested that the O2•− level in HSCs was positively correlated with the immunosuppressive ability of the HCC microenvironment.
a, b O2•− imaging and immunofluorescence staining of ordinary HCC mice and HCC mice with aHSCs. c, d O2•− imaging and immunofluorescence staining of HCC mice with the inhibited aHSCs by D-pen. λex = 800 nm, λem = 450–550 nm. Scale bar = 100 μm. The data were expressed as mean ± SD, n = 5. ***p < 0.001 compared to the control group.
It is important that the tumor volume and weight of aHSCs–HCC mice were significantly higher than those of control HCC mice. Meanwhile, using D-pen or VE to reduce the degree of HSCs activation could obviously inhibit the growth of group tumor (Supplementary Fig. 21). This suggests that aHSCs can promote tumor growth. To prove that immune evasion is primarily induced by ROS production, we performed experiments to validate the role of ROS on immune evasion. The experimental results showed that the tumor volume and weight of the mice in the experimental group were smaller compared to the control group (Supplementary Fig. 22). This indicates that scavenging ROS can inhibit tumor growth to a certain extent. All in all, we found that the excess O2•− generated by the aHSCs enhanced the immunosuppressive ability of the HCC microenvironment, which in turn affects the growth and development of tumor in mice.
Mechanism of immune evasion mediated by HSCs O2 •− in HCC
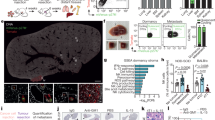
Inspired by the above-mentioned exciting findings, we explored the molecular mechanism by which O2•− regulates the immune evasion of HCC. In view of upregulation of PD-L1 resulting in immune evasion, we first investigated the expression of PD-L1 during the activation of HSCs. As shown in Fig. 7, we found an obvious upregulation of PD-L1 after HSCs were activated in orthotopic mouse models of HCC. Meanwhile, inhibiting the activation of HSCs led to a distinctly downregulation of PD-L1. Collectively, these results demonstrated that PD-L1 was involved in aHSCs-mediated immune evasion of HCC. The PD-L1 blocking antibody treatment experiments results showed that the tumor volume and weight of mice in the PD-L1 group were significantly reduced (Supplementary Fig. 23). This result suggests that the use of PD-L1 inhibitors can significantly inhibit the growth of tumor in mice, revealing the essential role of PD-L1 in immune evasion.
Next, we explored the signaling pathway leading to the expression change of PD-L1 in aHSCs. Previous studies have indicated that CDK4 could regulate the expression of PD-L1 through the CDK4/p-FZR1/SPOP/PD-L1 mechanism31. Therefore, we analyzed the effects of changes in HSCs status on CDK4 activity and related molecular levels. Compared with control HCC mice, we noted that CDK4 activity and p-FZR1 levels were decreased after HSCs activation. Low p-FZR1 levels could induce SPOP protein degradation and ultimately promote the upregulation of PD-L1 level (Fig. 7a). In addition, inhibition of HSCs activation could increase CDK4 activity and decrease PD-L1 expression level (Fig. 7b). Similarly, clearance of ROS produced by hepatic stellate cell activation can regulate CDK4 activity and PD-L1 levels. These results elucidated that the mechanism of CDK4 mediating PD-L1 was regulated by the activity of HSCs.
However, what cause the change of CDK4 activity remains unknown. Previous reports suggest that O2•− can damage protein activity by oxidizing amino acid residues17. Therefore, we hypothesized that O2•− in aHSCs might damage CDK4 activity, thereby promoting the immune evasion of HCC. To put our hypothesis to the test, we conducted proteomic analyses through LC–MS/MS to investigate the post-translational modifications of CDK4 by O2•−. As shown in Fig. 8, three histidine residues (H30, H68, and H95)32,33 in the active region of CDK4 were oxidized, which should be responsible for the inactivation of CDK4. Based on the above experimental results, we finally proposed a new detailed mechanism by which HSCs promote the immune evasion of HCC. The excess O2•− produced by aHSCs damaged the activity of CDK4, increased the level of PD-L1, and finally promoted the immune evasion of HCC.
Discussion
The immunomodulatory role played by HSCs during HCC development has been widely discussed. It is known that HSCs can produce large amounts of ROS when activated, but little is known about their role. Based on the important impact of ROS in cancer development, we analyzed the role of ROS within HSCs in the immune evasion of HCC. Under oxidative stress conditions, the intracellular electron transport chain generates the first ROS-O2•−, which is a precursor of several ROS and has a remarkable influence in biological signal transduction. However, it is not clear about how O2•− within HSCs affects downstream bioactive molecules in relation to immune evasion from HCC. Utilizing the probe TPH, we observed a positive correlation between the level of O2•− in HSCs and immune evasion of HCC. This finding provides strong evidence that ROS in HSCs promotes immune evasion from HCC.
It has been reported that activated-HSCs are able to help tumor cells immune evasion by upregulate PD-L1, but its regulatory mechanism is unclear. Meanwhile, some researchers have found a link between ROS and PD-L1. Using fluorescence imaging, the elevated levels of O2•− were found in cells and in vivo. This result prompted us to explore whether O2•− within HSCs can regulate the expression of PD-L1 during oxidative stress. Therefore, we analyzed the relationship between O2•− and PD-L1 in detail. The results of western blot experiments displayed that inducing O2•− overproduction could increase the level of PD-L1. Meanwhile, excess O2•− could significantly promote the growth of HCC in mice. On the contrary, the level of PD-L1 was significantly reduced and the growth of HCC was effectively delayed by decreased O2•−. In conclusion, we found that O2•− within HSCs can upregulate intracellular PD-L1, which ultimately leads to immune evasion from HCC.
It was found that the expression of PD-L1 was regulated by CDK4. Numerous experiments have shown that excess O2•− can damage DNA and disrupt protein structure, thus affecting the normal physiological functions of organisms. We were interested in whether O2•− regulates CDK4 activity. The western blot experiments revealed that excess O2•− could reduce the levels of molecules downstream of CDK4. In contrast, the phenomenon disappeared when O2•− was removed. The above results suggest that excess O2•− within HSCs can increase PD-L1 levels by decreasing CDK4 activity. The results of proteomics analysis experiments further indicated that excess O2•− could oxidize amino acid residues in the CDK4 active site. These data convincingly suggest that O2•− in HSC induces upregulation of PD-L1 levels by impairing CDK4 activity, and eventually lead to immune evasion. In conclusion, we provided a signaling pathway for the immune evasion of HCC mediated by O2•− within HSCs.
In order to explore the role of O2•− in HSCs in the immune evasion of HCC, we developed a two-photon fluorescence probe TPH for specific imaging detection of O2•−. Using TPH, we observed the increase of O2•− during HSCs activation in cells and in vivo. Notably, we found that the immunosuppressive ability of the HCC microenvironment was correlated with the activation status of HSCs. Further studies indicated that the excess O2•− generated HSCs modified the functional regions of CDK4, resulting in the inactivation of CDK4. Subsequently, the phosphorylation of FZR1 decreased, and caused to more SPOP degraded. The decrease of SPOP led to the increase of PD-L1 in HCC mice, conclusively inducing immune evasion (Fig. 9). This work provided a molecular mechanism by which O2•− in HSCs mediated immune evasion from HCC. We propose that this work can provide a supplement to understanding the occurrence and development of HCC. Meanwhile, it is expected to provide new ideas for immunotherapy methods for HCC.
Methods
Ethical statement
All animal care and experimental protocols complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China and were approved by the Animal Care Committee of Shandong Normal University (AEECSDNU2022023).
Measurement of two-photon cross sections
The two-photon absorption cross sections δ were determined using the femtosecond (fs) fluorescence measurement technique. TPH was dissolved in cell lysis buffer at a concentration of 40 μM, and then the TP-excited fluorescence spectra were measured at 800 nm. Fluorescein (1.0 × 10−4 M, pH = 11) whose TP properties have been well characterized in the literature, was used as the reference. The value of δ was calculated using the following equation.
The subscripts s and r refer to the sample and the reference material, respectively. δ is the TP absorption cross-sectional value, C is the concentration of the solution, n is the refractive index of the solution, F is the TP-excited fluorescence intensity, and Φ is the fluorescence quantum yield.
Cell culture
LX-2 cells were purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, China), and cultured in DMEM medium with 10% fetal bovine serum, 1% streptomycin, and 1% penicillin. The cells were placed in an MCO-15AC incubator (Sanyo, Tokyo, Japan) with culture parameters of 37 °C, 5% CO2, and 95% air. Two days before the cell imaging experiment, we digested the cells with trypsin and put an appropriate amount of cells into the culture dish for the convenience of the subsequent experimental operation.
Cytotoxicity assays
The cytotoxicity of 2-(2-methoxy-4-nitrobenzene)-3-(4-nitrobenzene)-5-(2,4-disulfonybenzene)-2h-tetrazole monosodium salt (CCK8) was tested. LX-2 cells were inoculated on 96-well plates with a concentration of 1 × 105 cell pore−1 and placed in an incubator for 24 h. Then, cells were incubated with different concentrations of probes for 12 h. The cells were rinsed with PBS, followed by 10 µL CCK8 solution and 90 µL medium were added to per well. After 1 h, the absorbance at 450 nm was measured by enzyme-labeled instrument, and the survival rate of cells was calculated.
Mice
All male C57 mice (age: 4–6 weeks; average weight: 20 ± 2 g) were purchased from Jinan Pengyue Experimental Animal Breeding Company Limited. The purchased 4–6 weeks C57BL/6 mice were placed in the animal house with 12 h light to ensure adequate water and food for the mice. After a week of adaptation, the mice were randomly grouped to construct mouse models of HSCs with different activation degrees and liver cancer models of HSCs with different activation degrees, respectively.
The mouse models with various levels of aHSCs
Mice were intraperitoneally injected with olive oil or CCl4 solution (1.0 mL kg−1) twice a week for 2, 4, and 6 weeks, respectively. Mice in the treatment group were injected with D-pen (100 mg kg−1) or vitamin E (100 mg kg−1) twice a week for 2 weeks after 6 weeks of CCl4 solution.
The orthotopic mouse models of HCC containing quiescent-HSCs or aHSCs
Mice were intraperitoneally injected with olive oil or CCl4 solution (1.0 mL kg−1) twice a week for 6 weeks, and then the orthotopic mouse models of HCC were constructed. Mice in the treatment group were injected with D-pen (100 mg kg−1) or VE (100 mg kg−1) for 2 weeks after 6 weeks of CCl4 solution, and then the orthotopic mouse models of HCC were constructed.
Fluorescence imaging experiments in vivo
In vivo imaging experiments, TPH was first injected intraperitoneally into model mice with 0.62 mg kg−1. After 30 min, the mice were injected intraperitoneally to numb them, and the mice were opened. The fluorescence imaging was performed using a two-photon fluorescence imaging microscope at an excitation wavelength of 800 nm.
Proteomic analysis
The CDK4 was dissolved in the appropriate buffer (50 mM Tris, pH = 8.0). Then, 1 mM superoxide anion (O2•−) was added, and incubated at 37 °C for 2 h. CDK4 was trypsin digested, and the peptides were isolated from the hydrolysate by solid phase extraction on the C-18 column. Proteomic analysis was performed by LC–MS/MS.
Statistics and reproducibility
The data were analyzed using Origin Pro 2021 (64-bit). Student’s t test was used to evaluate the differences between the data. P values < 0.05 were considered statistically significant. It was defined as *P < 0.05, **P < 0.01, and ***P < 0.001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data generated in this study are provided in the Supplementary Information/Source Data file. There are no restrictions on data availability in the current work. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository34,35 with the dataset identifier PXD051328.
References
Craig, A. J., Von Felden, J., Garcia-Lezana, T., Sarcognato, S. & Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 17, 139–152 (2020).
Wang, C., Cao, Y., Yang, C., Bernards, R. & Qin, W. Exploring liver cancer biology through functional genetic screens. Nat. Rev. Gastroenterol. Hepatol. 18, 690–704 (2021).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172 (2022).
Sangro, B., Sarobe, P., Hervás-Stubbs, S. & Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 525–543 (2021).
Rizvi, S., Wang, J. & El-Khoueiry, A. B. Liver cancer immunity. Hepatology 73, 86–103 (2021).
Molina-Sánchez, P. et al. Cooperation between distinct cancer driver genes underlies intertumor heterogeneity in hepatocellular carcinoma. Gastroenterology 159, 2203–2220 (2020).
Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Friedman, S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669 (2008).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397–411 (2017).
Myojin, Y. et al. Hepatic stellate cells in hepatocellular carcinoma promote tumor growth via growth differentiation factor 15 production. Gastroenterology 160, 1741–1754 (2021).
Coulouarn, C. et al. Hepatocyte–stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma hepatocyte–stellate cell cross-talk in liver cancer. Cancer Res. 72, 2533–2542 (2012).
Han, S. et al. Activated hepatic stellate cells promote hepatocellular carcinoma cell migration and invasion via the activation of FAK-MMP9 signaling. Oncol. Rep. 31, 641–648 (2014).
Xiao, H. et al. Versatile fluorescent probes for imaging the superoxide anion in living cells and in vivo. Angew. Chem., Int. Ed. 132, 4244–4258 (2020).
Zhang, Y. et al. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 23, 898–914 (2013).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Ding, Q. et al. Oxidative damage of tryptophan hydroxylase-2 mediated by peroxisomal superoxide anion radical in brains of mouse with depression. J. Am. Chem. Soc. 142, 20735–20743 (2020).
Zhang, W. et al. Dynamic and reversible fluorescence imaging of superoxide anion fluctuations in live cells and in vivo. J. Am. Chem. Soc. 135, 14956–14959 (2013).
Guo, Z. et al. Specific hepatic stellate cell-penetrating peptide targeted delivery of a KLA peptide reduces collagen accumulation by inducing apoptosis. J. Drug Target. 25, 715–723 (2017).
Huang, P., Feng, L., Oldham, E. A., Keating, M. J. & Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407, 390–395 (2000).
Zhou, Y., Hileman, E. O., Plunkett, W., Keating, M. J. & Huang, P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood J. Am. Soc. Hematol. 101, 4098–4104 (2003).
Du, Y., Miller, C. M. & Kern, T. S. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic. Biol. Med. 35, 1491–1499 (2003).
Kharbanda, K. K. et al. Transforming growth factor-β induces contraction of activated hepatic stellate cells. J. Hepatol. 41, 60–66 (2004).
Trautwein, C., Friedman, S. L., Schuppan, D. & Pinzani, M. Hepatic fibrosis: concept to treatment. J. Hepatol. 62, S15–S24 (2015).
Epstein, O. et al. D-penicillamine treatment improves survival in primary biliary cirrhosis. Lancet 317, 1275–1277 (1981).
Vilar‐Gomez, E. et al. Vitamin E improves transplant‐free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology 71, 495–509 (2020).
Xu, Q. et al. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 291, 20030–20041 (2016).
Jackson, S. H., Devadas, S., Kwon, J., Pinto, L. A. & Williams, M. S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 5, 818–827 (2004).
Kwon, J. et al. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 3, ra59 (2010).
Devadas, S., Zaritskaya, L., Rhee, S. G., Oberley, L. & Williams, M. S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195, 59–70 (2002).
Zhang, J. et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018).
Jeffrey, P. D., Tong, L. & Pavletich, N. P. Structural basis of inhibition of CDK–cyclin complexes by INK4 inhibitors. Genes Dev. 14, 3115–3125 (2000).
Day, P. J. et al. Crystal structure of human CDK4 in complex with a D-type cyclin. Proc. Natl Acad. Sci. USA 106, 4166–4170 (2009).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22134004, 22074083, 21927811), the Key Research and Development Program of Shandong Province (2018YFJH0502), National Science Foundation of Shandong Province of China (ZR2020ZD17), Youth Fund of Natural Science Foundation of Shandong Province (ZR2021QB042), Taishan Scholars (Tsqn201812125), National Youth Qihuang Scholar Training Program, Shandong Province Traditional Chinese Medicine High Level Talent Cultivation Project and Key Research and Development Project of Shandong Province (2020CXGX010505).
Author information
Authors and Affiliations
Contributions
X.W., P.L., and B.T. conceived the work. Y.M. and C.W. performed optical characterization experiments and mouse experiments. F.Z. and X.Q. performed cell experiments. X.L. provided technical support. Y.M. and C.W. wrote the original draft. X.W., P.L., and B.T. reviewed and finished the final editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Periklis Pantazis, Zhijuan Qiu, and Dario Ummarino.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, Y., Wu, C., Wang, X. et al. Fluorescence imaging sheds light on the immune evasion mechanisms of hepatic stellate cells mediated by superoxide anion. Commun Biol 7, 558 (2024). https://doi.org/10.1038/s42003-024-06245-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06245-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.