Abstract
Most functional eukaryotic mRNAs contain a 5′ 7-methylguanosine (m7G) cap. Although capping is essential for many biological processes including mRNA processing, export and translation, the fate of uncapped transcripts has not been studied extensively. Here, we employed fast nuclear depletion of the capping enzymes in Saccharomyces cerevisiae to uncover the turnover of the transcripts that failed to be capped. We show that although the degradation of cap-deficient mRNA is dominant, the levels of hundreds of non-capped mRNAs increase upon depletion of the capping enzymes. Overall, the abundance of non-capped mRNAs is inversely correlated to the expression levels, altogether resembling the effects observed in cells lacking the cytoplasmic 5′−3′ exonuclease Xrn1 and indicating differential degradation fates of non-capped mRNAs. The inactivation of the nuclear 5′−3′ exonuclease Rat1 does not rescue the non-capped mRNA levels indicating that Rat1 is not involved in their degradation and consequently, the lack of the capping does not affect the distribution of RNA Polymerase II on the chromatin. Our data indicate that the cap presence is essential to initiate the Xrn1-dependent degradation of mRNAs underpinning the role of 5′ cap in the Xrn1-dependent buffering of the cellular mRNA levels.
Similar content being viewed by others
Introduction
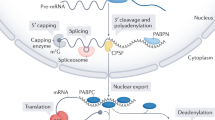
The N7-methylated guanosine (m7G cap) linked to the first nucleotide of the RNA molecule, is a hallmark structure of eukaryotic transcripts generated by the RNA Polymerase II (Pol II). The cap is involved in various steps of mRNA turnover, processing, transport and translation and therefore, essential for cell viability1, 2. Many of these biological functions are related to the complexes interacting with the m7G caps: the nuclear cap-binding complex (CBC) and the cytoplasmic eukaryotic initiation factor 4E (eIF4E)3,4,5. The biosynthesis of the m7G cap requires three enzymes: the RNA triphosphatase (TPase) which removes the γ-phosphate from the RNA 5′ end; the RNA guanylyltransferase (GTase) which transfers a GMP group to the diphosphate 5′ end; and the guanine-N7 methyltransferase adding a methyl group to the N7 amine of the guanine cap6. This synthesis pathway of the m7G cap is conserved in eukaryotes, however, the enzymes involved vary between the organisms7. In humans and other higher eukaryotes, a bifunctional protein RNGTT acts as RNA 5′-triphosphatase and guanylyltransferase while the methylation is mediated by a separate protein RNMT8. In Saccharomyces cerevisiae, three proteins are responsible for RNA capping: the 5′-triphosphatase Cet1 and the guanylyltransferase Ceg1 work together as a stable heterodimer or heterotetramer, while the methyltransferase Abd1 acts independently9,10,11. The capping enzymes are recruited to the Pol II at the early stage of transcription. In S. cerevisiae, Cet1 and Ceg1 bind the serine 5 phosphorylated C-terminal domain (CTD) of Pol II at the transcription start site (TSS)9,12. When the nascent RNA reaches the length of ~17 nt, the Ceg1-Cet1 complex docks to the Pol II surface close to the RNA exit tunnel11 and adds the terminal guanosine as soon as the first 25–30 nt of the nascent transcript extrudes from the transcribing complex13. Changes in the phosphorylation status of the Pol II CTD promoting transcription elongation result in the rapid dissociation of Ceg1-Cet111. This and the increased phosphorylation of CTD Ser2 recruit Abd1 about 110 nt downstream of the TSS12 to complete the formation of the m7G cap.
If the methylation of the added 5′ guanosine residue fails, the defective transcripts are detected by Npl3-mediated surveillance mechanism that triggers decapping by Rai1 and subsequent RNA degradation by its co-factor, the 5′−3′ exonuclease Rat114,15. The binding of CBC to the cap, which indicates correct and completed capping, disrupts Npl3-Rai1 and prevents degradation15. Nascent transcripts lacking the 5′ cap may be co-transcriptionally degraded. Such a process occurs at the 3′ ends of genes where cleavage over the poly(A) signal in the nascent RNA creates an unprotected 5′ monophosphate end which serves as an entry point for Rat116,17,18. If the degradation rate exceeds the RNA synthesis rate, Rat1 reaches the transcribing complex and Pol II is displaced from DNA by so-called ‘torpedo’ termination16,17,18,19. A similar mechanism has been suggested to occur upstream of the polyA site when capping fails at the 5′ end of the gene. Analysis of the FMP27 gene in S. cerevisiae ceg1-63 mutant revealed that the transcription of uncapped pre-mRNA is prematurely terminated by Rat1 within the gene body which reduced the level of FMP27 mRNA20. Analysis of the temperature-sensitive mutants showed that the inactivation of Ceg1 also affected the accumulation of heat-shock induced SSA1 and SSA4 mRNAs. Their levels were restored by the deletion of the predominantly cytoplasmic 5′−3′ exonuclease Xrn11. Xrn1 mediates global mRNA degradation and acts with the decapping factors as a sensor of the cellular mRNA levels that controls and regulates the homoeostatic mRNA abundance21,22.
Here, we investigated the consequences of the capping absence on RNA accumulation and transcription. We employed rapid nuclear depletion of Ceg1 to investigate the global turnover of non-capped mRNAs. We found that the lack of the capping resulted in the differential expression of cellular RNAs: the mRNA levels of highly expressed genes decreased while the mRNAs of genes expressed at lower levels increased indicating differential specificity of degradation pathways of highly and lowly expressed genes. The deletion of XRN1 resulted in a similar redistribution in the mRNA abundance which was not synergistically affected by the nuclear depletion of Ceg1. Our data indicate that m7G cap and Xrn1 act together in the control of the mRNA 5′−3′ degradation. In particular, the presence of the cap is critical for the formation of the correct 5′ end substrate for efficient Xrn1-dependent degradation. The non-capped mRNA levels were not rescued by the inactivation of the nuclear 5′−3′ exonuclease Rat 1 and the disruption of the 5′ end capping had a minor effect on the distribution of Pol II on the chromatin. Overall, our data indicate that non-capped mRNAs, in contrast to defectively capped mRNAs, are not specifically marked for degradation and that m7G caps play important roles in the Xrn1-dependent degradation buffering cellular mRNA levels.
Results
Differential expression of non-capped mRNAs
The impact of defective or complete lack of capping on RNA abundance has never been studied genome-wide before. In our experimental approach, we employed the anchor-away (AA) system23 to deplete the capping enzymes from the nucleus and rapidly affect the cap formation. FRB tag fused with either Cet1, Ceg1 or Abd1 (cet1-AA, ceg1-AA and abd1-AA strains) in the presence of rapamycin heterodimerized with FKBP12 attached to the ribosomal protein Rpl13a, which was exported to the cytoplasm thus removing the capping enzymes from the nucleus. Since rapamycin affects the TOR signalling pathway24 the strain used for this system carries the tor1-1 mutation, which permits normal growth on rapamycin23. The nuclear depletions of Cet1 and Ceg1 were lethal (Fig. 1a) indicating the efficacy of the method as cap formation is essential for cell viability25,26. The inactivation of Abd1 activity has been reported to be non-viable27, however, the nuclear depletion of Abd1 had a mediocre impact on cell growth (Supplementary Fig. 1a). This indicates that Abd1 may still perform its functions after the relocation to the cytoplasm. The nuclear depletion of either Cbp20 or Cbp80 was viable, consistent with the fact that the CBC is not essential in S. cerevisiae (Fig. 1a). In our functional experiments, the same yeast culture was split into a medium containing either vehicle DMSO or rapamycin which represented the WT (DMSO) or the nuclear depletion (Rap) condition (Fig. 1b). First, we tested the efficiency of Ceg1 and CBC subunit depletions. We confirmed by immunofluorescence visualisation of GFP-fused proteins that proteins were depleted from the nucleus after 45 min of rapamycin treatment (Fig. 1c, d). Next, we confirmed that RNA capping was indeed affected by the nuclear depletion of Ceg1. We co-immunoprecipitated RNA isolated from the ceg1-AA strain growing for 45 min on either DMSO or Rap with the anti-m7G cap antibody (H-20) and tested mRNAs levels using qPCR (Fig. 1e and Supplementary Fig. 1b). The amount of capped mRNAs (normalised to human mRNA GAPDH spike) was reduced up to 80% in ceg1-AA (growing on Rap) when compared to WT (control cells grown on DMSO). The nuclear depletion of either Ceg1 or Cet1 (ceg1-AA or cet1-AA) resulted in a similar decrease in the ADH1 mRNA (which has been used in many studies as a model yeast mRNA) (Fig. 1f and Supplementary Fig. 1c); therefore, in the subsequent analyses, we focused mainly on the effects of Ceg1 nuclear removal. We also confirmed that the nuclear depletion of CBC did not affect ADH1 mRNA and so the effect we observed during the depletion of capping machinery was solely caused by the lack of Ceg1/Cet1 function (Fig. 1f and Supplementary Fig. 1c). Nuclear depletion of Abd1 did not change ADH1 levels (Supplementary Fig. 1d).
a Spot test showing the growth of the AA strains. Serial dilutions of each strain were spotted on YPD media containing DMSO or rapamycin (1 μg/ml). b The outline of the experimental approach using AA strains. Cell cultures at the exponential phase were split and supplemented with DMSO (WT; control sample) or rapamycin (Rap; nuclear depletion sample). c Fluorescent microscopy localisation of GFP-AA tagged strains after 45 min incubation on DMSO or Rap. DAPI was used to visualise nuclei. White scale bars represent 2 μM. d Quantification of mean signals intensity of the cytoplasm and nucleus in GFP-AA strains growing on DMSO (D) or rapamycin (R). Statistical significance was determined through one way ANOVA. ** denotes p < 0.01, **** denotes p < 0.0001. e Quantification of the capped RNA by RT-qPCR after 45 min of rapamycin treatment compared to control (DMSO; WT). The RNA isolated from yeast was spiked-in with capped human RNA and immunoprecipitated by the anti-m7G cap antibody (clone H20). The RNA levels were normalised to human GAPDH mRNA. The error bars show standard deviation of three independent experiments. f RT-qPCR analysis of the ADH1 mRNA levels in the AA-tagged strains after 45 min of rapamycin treatment compared to the control (isogenic strains on DMSO) set to 1 (dotted line). The error bars show standard deviation of two (cbp20-AA and cbp80-AA) or three independent experiments g RNA-seq analysis showing differential expression of mRNA species in ceg1-AA (Rap) strain (-cap) after 45 min of rapamycin treatment compared to the control condition (WT). Increased (Ic) RNA species with log2 fold change >1 are labelled in red. Decreased (Dc) RNA species showing log2 fold change <-1 are in blue. The average values with padj < 0.05 of n = 2 experiments are shown, the zero-change line is in green and the ±1 log2 fold change threshold is indicated with black lines. The changes in levels of decreased (h) and increased (i) mRNAs over time during nuclear depletion of Ceg1. RT-qPCR analysis of RNA levels for different genes measured at 0, 45, 90 and 120 min of rapamycin treatment relative to control (WT) set to 1 (dotted line). The error bars show standard deviation of three independent experiments.
Next, we nucleodepleted Ceg1 for 45 min and performed a global analysis of mRNA levels. Our RNA-seq analysis identified 1205 mRNAs affected by the nuclear depletion of Ceg1 (displaying log2 fold change <−1 and >1) (Fig. 1g). 736 (61%) of these mRNAs decreased while the levels of 469 genes (39%) increased when compared to the control (WT). Next, we tested how the mRNA levels changed over time during the nuclear depletion of Ceg1. We selected ADH1, PYK2 and PMA1 genes whose levels decreased upon Ceg1 depletion in the RNA-seq data. RT-qPCR analysis revealed that their mRNAs decreased even further after 90 min and stabilised after 120 min (Fig. 1h). We also tested PML39, STP2 and CTA1 whose levels increased upon Ceg1 depletion (Fig. 1i). PML39 and STP2 mRNAs peaked after 90 min, while the accumulation of CTA1, displayed a constant increase at all time points. The increased and decreased mRNA levels were confirmed by RT-qPCR relative to exogenous spike-in control (Supplementary Fig. 1e). Yeast RNA was mixed 1:1 with human RNA and subjected to qPCR analysis where mRNA levels were normalised to 28S rRNA and reverse transcription reaction efficiency was corrected to human GAPDH.
Accumulation of non-capped mRNAs depends on individual expression levels
To understand the differential expression observed upon depletion of the capping machinery, we analysed the features of affected mRNAs. First, we tested the basal expression (levels in WT cells) of the differentially expressed mRNAs in Ceg1-depleted cells. We found that increased mRNAs were generally lower expressed (median transcript per million TPM log2 = 2.7) than the decreased mRNAs (median log2 TPM = 6.2) (Fig. 2a). Next, we split all 1205 protein-coding genes differentially expressed in ceg1-AA into three bins according to their expression levels in WT cells: the top 25% were classified as highly expressed (High), the bottom 25% as lowly expressed (Low) and the remaining 50% as medially expressed (Mid). Most of the lowly expressed genes increased their mRNA levels (291 out of 301), while most of the highly expressed mRNAs decreased (300 out of 301) after the Ceg1 depletion (Fig. 2b, c). Mid-range expressed mRNAs were the least affected by the depletion; however, they displayed a tendency to decrease in Ceg1-depleted cells (426 decreased versus 176 increased), correlating with increasing expression of individual genes. This was also evident at the single gene level as shown for representative ZRT1, STB4 and ADH1 (Fig. 2d). Next, we employed available datasets estimating transcription rates (TR) and Pol II density in S. cerevisiae28 and applied them to the sets of differentially expressed mRNAs identified in the ceg1-AA. These analyses revealed that mRNAs which increased upon Ceg1 have lower transcription rates and Pol II density (Fig. 2e and Supplementary Fig. 2a, b) than genes whose mRNAs decreased in Ceg1-depleted cells. Consistently with the fact that shorter genes are generally associated with higher expression, the mRNAs accumulating in ceg1-AA were also longer than those from the decreased set (average gene length 1430 vs 1113 nt) (Supplementary Fig. 2c) additionally confirming the relation between the expression strength and stability of non-capped RNAs. The determination of the half-lives of affected mRNAs would additionally characterise these transcripts. However, available datasets estimating mRNA half-lives in yeast significantly vary depending on the method used in the studies (Supplementary Fig. 2d)29,30, therefore such analysis was not conclusive.
a Differentially expressed mRNAs in ceg1-AA (Rap) vs ceg1-AA (DMSO/WT) sorted by their expression levels: Increased (Ic) or decreased (Dc). The expression levels were calculated by the log2 of Transcript Per Million (log2 TPM) value in WT (ceg1-AA on DMSO). The asterisks (*) indicate the statistical significance calculated via t test. b The distribution of differentially expressed mRNAs in ceg1-AA (Rap) depending on their basal expression levels in WT cells. c The overall fold change for differentially expressed mRNAs in ceg1-AA (Rap) vs ceg1-AA (DMSO) ranked according to their basal expression levels. The asterisks (*) indicate the statistical significance calculated via ANOVA. d Genome viewer tracks showing RNA-seq reads in ceg1-AA (DMSO) and ceg1-AA (Rap) for lowly (red), medial (grey) and highly (blue) expressed genes. e Box plots showing transcription rates and Pol II density for differentially expressed mRNAs in ceg1-AA (Rap) vs ceg1-AA (DMSO). The asterisks (*) indicate the statistical significance calculated via t test. f The accumulation of the NEL025c (left) and SUT238 (right) RNAs in ceg1-AA strain upon 0, 45, 90 and 120 min of rapamycin treatment compared to WT (DMSO) set to 1 (dotted line). Analysis of SUT238 n = 2, individual points of biological replicates are shown. g RT-qPCR analysis showing the levels of ADH1 mRNA and NEL025 ncRNA upon 6-AU (red) and NH4OH (blue) treatments in Ceg1-depleted cells. The error bars show the standard deviation of three independent experiments. Statistical analysis is shown for the last time point (120 min). a, c, e ns = P > 0.05; * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001; **** = P ≤ 0.0001. The box limits show the first and the third quartile (Q1 and Q3). The line inside the box represents the median value. The lines (whisker) show the maximum and minimum value within 1.5 times interquartile Q1 and Q3. The minimum/maximum whisker values are calculated as Q1/Q3 ∓ 1.5 * IQR (interquartile range). Each point represents the value relative to a single gene.
We also investigated Pol II-transcribed non-coding RNAs (ncRNAs), called cryptic unstable transcripts (CUTs) and stable unannotated transcripts (SUTs), which in normal conditions are expressed at a very low level31, 32. Most CUTs are detectable only upon the inactivation of the 3′−5′ exonuclease Rrp6 subunit of the nuclear exosome33 and for this reason, only a handful of CUTs were detected in our RNA-seq analysis of the ceg1-AA strain. Thus, we focused our investigation on the well-studied ncRNA CUT542 (NEL025c). The nuclear depletion of Ceg1 increased the levels of NEL025c (Fig. 2f) up to ten times after 120 min of rapamycin treatment, consistent with the fact that lowly expressed Pol II transcripts accumulate when capping enzymes are not functional. A similar effect was observed for SUT238 (Fig. 2f).
Next, we tested whether the alternation in the RNA synthesis could rescue the levels of ADH1 mRNA and NEL025c ncRNA in ceg1-AA. We affected Pol II elongation rate34,35, by treating cells with 6-azauracil (6-AU) for 30 min, which causes depletion of intracellular nucleotide pools36. The treatment was followed by Ceg1 nuclear depletion and analysis of the ADH1 and NEL025c levels relative to the WT (Fig. 2g). Slower RNA synthesis stabilised ADH1 mRNA however, did not affect the overexpression of NEL025c which suggests that the low expression of this gene reached the threshold where further decrease had minimal impact on the accumulation of non-capped RNA.
Highly expressed genes are characterised not only by the strength of the promoter but also their sequences which determine the efficiency of translation or degradation pathways. The set of genes that decreased upon Ceg1 nuclear depletion displayed a higher GC content than the increased set (Fig. 3a) as the high GC content is associated with high expression37. Moreover, we found that the increased and decreased mRNA groups also display a different codon bias in their sequences (Fig. 3b). This is consistent with the fact that codon usage for highly expressed genes has a high correlation with tRNA abundance and thus differs from the overall characteristics of the whole yeast genome38 (Supplementary Fig. 3). Both features may contribute to the differential fate of the transcripts, including RNA storage and selection of the degradation pathways39,40,41. For example, decapping enzymes and Xrn1 preferentially bind transcripts containing a low number of optimal codons while the deadenylation enzymes and the 3′−5′ exonucleolytic complex exosome bind more strongly to codon optimal transcripts40. Finally, utilising Multiple Em for Motif Elicitation (MEME, https://meme-suite.org/meme/) analysis identified the sequence motif (A/G)GAAAA strongly enriched and uniformly distributed in the upregulated mRNAs (Fig. 3c). The motif resembles the binding sequence of the human NFAT (nuclear factor of activated T-cells) factor involved in T-cells immune response42, displaying functional similarity with the transcription factor Crz1 in S. cerevisiae43. However, the function of the identified sequence in the context of RNA stability remains unknown.
a The GC content of increased (Ic) and decreased (Dc) mRNAs upon Ceg1 nuclear depletion. The GC content percentage distribution frequency is shown as a density plot. The statistical significance was assessed via Chi-squared and Student’s t test. ns = P > 0.05; * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001; **** = P ≤ 0.0001.The vertical dotted line denotes the median GC content of the yeast genome. b The bias in codon usage between Increased (Ic) and decreased (Dc) genes differentially expressed upon nucleodepletion of Ceg1 for 45 min. The plot shows the Measure Independent of Length and Composition (MILC) distance between the two sets of genes. c Logo sequence overrepresented in the increased mRNAs in ceg1-AA (Rap).
Minor contribution of Rat1 in the degradation of non-capped Pol II transcripts
Next, we tested how non-capped mRNAs are degraded. Previous reports showed that mutation of the exonuclease Rat1 can delay degradation of FMP27 and GAL1 mRNAs in capping-deficient cells20 and that the Rai1/Rat1 complex is involved in the degradation of mRNAs with unmethylated caps14. Thus, we investigated if Rat1 inactivation could rescue the levels of non-capped mRNAs. We employed the same experimental system, ceg1-63 and ceg1-63/rat1-1 temperature-sensitive mutants, to assess the levels of highly expressed ADH1 as well as GAL1 and FMP27 genes previously used to study capping-dependent RNA degradation20. FMP27 is a lowly expressed gene placed under control of the strong inducible GAL1 promoter in the ceg1-63 strain20. We shifted the mutants and the parental strain to a non-permissive temperature (37 °C) for up to 120 min, for the analysis of GAL1::FMP27 the strain grew on galactose-containing medium. The mRNA levels of both ADH1 and GAL1::FMP27 decreased in ceg1-63 however, the inactivation of Rat1 in ceg1-63/rat1-1 did not rescue their abundance (Fig. 4a). The profile of the FMP27 levels expressed from the GAL1 promoter were mirrored by the levels of endogenous GAL1 mRNA (Fig. 4a) confirming that the mRNA levels were correlated with the strength of RNA synthesis in ceg1-63. Notably, FMP27 is expressed at a low level from its own promoter in WT cells and did not decrease in ceg1-AA but remained stable (Fig. 4b). We did not observe that mRNA levels in ceg1-63 were rescued by Rat1 inactivation at sub-permissive temperature (34 °C) (Supplementary Fig. 4a). Similar to mRNAs, the levels of lowly transcribed ncRNA NEL025c were not altered by the rat1-1 mutation in the ceg1-63 strain (Fig. 4a and Supplementary Fig. 4a). Although Rat1 may moderately delay degradation of non-capped mRNA20 our data indicate that Rat1 has rather minor role in the overall clearing of non-capped mRNAs.
a RNA levels of ADH1, FMP27, GAL1 and NEL025c in WT and temperature-sensitive mutants ceg1-63 and ceg1-63/rat1-1 after 0, 45, 90 and 120 min at a non-permissive temperature (37 °C) compared to control (WT at 25 °C) set to 1 (dotted lines). For analysis of GAL1:FMP27 and GAL1 mRNAs cells grew on galactose-containing medium. b FMP27 mRNA levels in ceg1-AA after 0, 45, 90 and 120 min of rapamycin treatment compared to WT (ceg1-AA on DMSO) set to 1 (dotted line). c ADH1 mRNA levels in ceg1-AA and ceg1-AA/rai1Δ after 45 min on Rap compared to isogenic controls growing on DMSO set to 1 (dotted line). d The distribution of total/unphosphorylated Pol II (total/unph Pol II) over FMP27 in control (DMSO) and after 45 min of rapamycin treatment (Rap) in ceg1-AA. The location of the amplicons used for the ChIP-qPCR over FMP27 is shown above the chart. The error bars show the standard deviation of three independent ChIP-qPCR experiments. The distribution of total/unphosphorylated Pol II (total/unph Pol II) as well as serine 5-phosphorylated (Ser5-P Pol II) and serine 2-phosphorylated (Ser2-P Pol II) Pol II isoforms over ADH1 (e) and PML39 (f) in control (DMSO) and after 45 min of rapamycin (Rap) in ceg1-AA strain. The location of the amplicons used for the ChIP-qPCR is shown above the charts. The error bars show the standard deviation of three independent ChIP-qPCR experiments. g The occupancy of the total/unphosphorylated Pol II at the 3′ end of ADH1, PYK2 and PMA1. Timepoints after 0, 45, 90 and 120 min on rapamycin compared to control (ceg1-AA on DMSO) and set to 1 (dotted line). The location of the amplicons used for the ChIP-qPCR is shown above the charts. The error bars show the standard deviation of three independent ChIP-qPCR experiments.
To avoid metabolism-changing temperature shifts of ceg1-63/rat1-1 we tested how the deletion of the gene encoding the Rat1 binding partner Rai1 impacts RNA levels in ceg1-AA. Rai1 converts triphosphorylated RNA 5′ ends generated by Pol II into monophosphate 5′ RNA which are substrates for 5′−3′ exonucleases44, 45. Rai1 also stabilises and activates Rat1 by forming a stable Rai1-Rat1 complex and together with Npl3 control degradation of cap-defective mRNAs15,46,47,48. The Ceg1 nuclear depletion resulted in a decrease of ADH1 mRNA in strains with either functional or deleted RAI1 (Fig. 4c). This further confirmed that Rat1 may not be efficiently involved in the degradation of non-capped transcripts.
Previous studies reported that shifting the temperature-sensitive mutant ceg1-63 to sub-permissive temperature resulted in a premature transcription termination of the eight kb-long gene FMP27 via a mechanism associated with Rat1-dependent co-transcriptional degradation of nascent RNA20. However, this effect was not fully recapitulated in the ceg1-AA strain consistently with our data indicating that Rat1 is not efficiently involved in degradation of non-capped mRNAs. ChIP analysis using an antibody recognising Pol II CTD revealed only a modest reduction of Pol II occupancy ~0.5 kb from the transcription start site but not further downstream of the gene (Fig. 4d). Since the average gene length in yeast is 1.4 kb49 (Supplementary Fig. 4b), we investigated how transcription was affected in ceg1-AA on shorter genes.
We analysed the distribution of Pol II phosphoisoforms on highly and lowly expressed genes ADH1 and PML39, respectively, in ceg1-AA (Fig. 4e, f). Total Pol II was partially depleted mainly on the 5′ ends for both highly and lowly expressed genes. Since we used 8WG16 antibody recognising non-modified heptapeptides of the CTD, we speculate that this decrease of the Pol II signal may reflect prematurely terminated unphosphorylated or hypophosphorylated Pol II. Indeed, both ser5-P and ser2-P Pol II phosphoisoforms, which represent actively transcribing Pol II, were only marginally affected by Ceg1 depletion (Fig. 4e, f). A similar but less pronounced effect was observed for the ADH1 gene in cet1-AA (Supplementary Fig. 4c). Nuclear depletion of Abd1 did not affect the distribution of Pol II over ADH1 (Supplementary Fig. 4d). We also investigated if longer nuclear depletion of Ceg1 resulted in increased premature transcription termination. We shifted cells to the medium containing Rap for up to 120 min and tested the levels of Pol II over the 3′ end of high and mid-range expressed ADH1, PYK2 and PMA1 genes (Fig. 4g). Consequently, we observed only ~20% decrease in Pol II signal at 45 min which was not exacerbated by longer nuclear depletion of Ceg1.
XRN1 deletion reciprocates Ceg1 nuclear depletion on the RNA level
Next, we tested if ADH1 levels could be rescued in ceg1-AA by the inactivation of the other major 5′−3′ exonuclease, Xrn1. Indeed, the deletion of XRN1 restored the levels of the non-capped ADH1 mRNA in ceg1-AA (Fig. 5a). Note that the ceg1-AA/xrn1Δ strain was non-viable on Rap-containing medium (Fig. 5b) confirming that non-capped mRNAs were not functional in this strain. The rescue effect observed for ADH1 in ceg1-AA/xrn1Δ (Rap) prompted us to test whether the differential expression in ceg1-AA could be restored globally by the deletion of XRN1. We performed RNA-seq analysis on the ceg1-AA/xrn1∆ strain growing on either DMSO (xrn1Δ) or Rap for 45 min and found only 18 decreased non-capped mRNAs, 13 of which were also reduced in ceg1-AA (Fig. 5c, e). Surprisingly, the number of increased mRNAs was also significantly lower in ceg1-AA/xrn1∆ when compared to ceg1-AA as we detected only 23 upregulated mRNAs (Fig. 5c, d). The observation that XRN1 deletion rescues increased mRNAs in ceg1-AA was somewhat unexpected. To elucidate this, we tested mRNA expression in ceg1-AA/xrn1∆ prior to the nuclear depletion of Ceg1. We compared ceg1-AA/xrn1∆ (DMSO) to WT (ceg1-AA on DMSO) and found that XRN1 deletion results in a global change in the mRNA expression which was similar to the pattern observed upon Ceg1 nuclear depletion. RNA-seq analysis of ceg1-AA/xrn1∆ on DMSO (referred as xrn1Δ) revealed 543 upregulated and 605 downregulated mRNAs with log2 fold change <−1 and >1 (Fig. 6a). The upregulated genes were lowly expressed (median log2 TPM = 2.6) while the decreased fraction was highly expressed (median log2 TPM = 6.2) in WT cells (Fig. 6b). Similarly to the previous analysis, all of 1148 mRNAs differentially expressed in xrn1∆ were split into three bins according to their expression levels in WT cells (Fig. 6c, d). The majority of the lowly expressed mRNAs increased (270 out of 282), while most of the highly expressed mRNAs decreased (280 out of 282) in xrn1Δ. The number of mid-range expressed genes was equally affected (268 upregulated, 296 downregulated) however, the median of their expression levels was overall decreased. Consistently, upregulated genes displayed lower transcription rates and Pol II density than downregulated genes (Fig. 6e). Since the observation in xrn1Δ very closely resembles those in ceg1-AA on Rap, we tested if the effect of nuclear depletion of Ceg1 in xrn1∆ strain was masked by the pre-existing aberrant accumulation of mRNAs caused by the XRN1 deletion. We assessed how many differentially expressed mRNAs in ceg1-AA were also differentially expressed in xrn1∆ prior to nuclear depletion of Ceg1. We found that 190 out of 469 mRNAs increased and 264 out of 736 mRNAs decreased in ceg1-AA were also similarly affected in xrn1∆ (Fig. 6f). This overlap identified only genes with log2 fold change over the ±1 threshold in both data sets therefore, we also assessed the levels of mRNAs differentially expressed in ceg1-AA in the total mRNA fraction in xrn1∆. Generally, most of the increased and decreased mRNAs identified in ceg1-AA were similarly affected in xrn1∆ (Fig. 6g) displaying a mean log2 fold change of 1.28 for the increased and −1.01 for the decreased mRNAs (Fig. 6h). We also performed reverse analysis and tested the levels of differentially expressed mRNA in xrn1∆ in ceg1-AA (Fig. 6i). Both groups are accordingly increased or decreased in ceg1-AA by a mean log2 fold change of 1.2 and −1.1, respectively (Fig. 6j). These analyses indicate that XRN1 deletion and nuclear depletion of capping enzymes have very similar effect on mRNA accumulation. This suggests a common mechanism affecting mRNA levels and therefore explains why the Xrn1 and Ceg1 co-inactivation did not result in many differentially expressed mRNAs. We concluded that the lack of the cap at the 5′ ends of mRNAs is insufficient to initiate Xrn1-dependent degradation and the process of the cap removal is the key step marking mRNAs for 5′−3′ degradation. Finally, we compared the RNA-seq dataset from ceg1-AA (Rap) and ceg1AA/xrn1∆ (Rap) to exclude the genes affected by either XRN1 deletion or Ceg1 depletion. We identified 482 up- and 536 downregulated (Supplementary Fig. 5a), both groups are expressed on similar levels in WT cells (Supplementary Fig. 5b).
a ADH1 mRNA levels in ceg1-AA and ceg1-AA/xrn1Δ after 45 min on Rap compared to isogenic controls growing on DMSO set to 1 (dotted line). RT-qPCR, the error bars show the standard deviation of three independent experiments. b Spot test showing the growth of ceg1-AA/xrn1Δ on DMSO and rapamycin. Serial dilutions of each strain were spotted on YPD media containing DMSO or rapamycin (1 μg/ml). c Differential expression of mRNAs after 45 min of rapamycin treatment in ceg1-AA/xrn1∆ (-cap -Xrn1) compared to the control condition ceg1-AA/xrn1∆ on DMSO (-Xrn1). Decreased (Dc) RNA species showing log2 fold change <-1 are in blue. Increased (Ic) RNA species with log2 fold change >1 are labelled in red. The zero-change line is in green. The black lines represent the ±1 log2 fold change threshold. The average values with padj < 0.05 of n = 2 experiments are shown. Increased (Ic) (d) or decreased (Dc) (e) mRNAs in ceg1-AA (Rap) are labelled on the plot showing mRNA levels in ceg1-AA/xrn1∆ (Rap) compared to the control condition ceg1-AA/xrn1∆ (DMSO). The average values with padj < 0.05 of n = 2 experiments are shown.
a Differential levels of mRNAs in xrn1∆ (ceg1-AA/xrn1∆ on DMSO) compared to WT (ceg1-AA on DMSO). Increased (Ic) mRNAs with log2 fold change >1 are labelled in red, decreased (Dc) mRNAs showing log2 fold change <-1 are in blue. The average values with padj < 0.05 of n = 2 experiments are shown, the zero-change line is in green. The black lines represent the ±1 log2 fold change threshold. b Differentially expressed mRNAs in xrn1∆ (ceg1-AA/xrn1∆ on DMSO) sorted by their expression levels calculated by the log2 Transcript Per Million (log2 TPM) value in WT (ceg1-AA on DMSO). c The distribution of differentially expressed mRNAs in xrn1∆ depending on their expression levels in WT cells. d The overall fold change for differentially expressed mRNAs in xrn1∆ ranked according to their expression levels in WT cells. e Transcription rates and Pol II density for differentially expressed mRNAs in xrn1∆ (ceg1-AA/xrn1∆ on DMSO). f The number of overlapping increased (Ic) or decreased (Dc) mRNAs in xrn1∆ (ceg1-AA/xrn1∆ on DMSO) and ceg1-AA (Rap). g Increased (red) or decreased (blue) mRNA in ceg1-AA (Rap) are labelled on the plots showing mRNA levels in ceg1-AA/xrn1∆ (DMSO) compared to the control condition (WT). The average values with padj <0.05 of n = 2 experiments are shown. h The log2 fold change in xrn1∆ for the sets of mRNAs differentially expressed in ceg1-AA (Rap). i Increased (red) or decreased (blue) mRNAs in xrn1∆ are labelled on the plots showing mRNA levels in ceg1-AA (Rap) compared to the control condition (WT). The average values with padj <0.05 of n = 2 experiments are shown. j The log2 fold change in ceg1-AA (Rap) for the sets of mRNAs differentially expressed in xrn1∆. b, d, e, h, j The asterisks (*) indicate the statistical significance calculated via t-test or ANOVA. ns = P > 0.05; * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001; **** = P ≤ 0.0001. The box limits show the first and the third quartile (Q1 and Q3). The line inside the box represents the median value. The lines (whisker) show the maximum and minimum value within 1.5 times interquartile Q1 and Q3. The minimum/maximum whisker values are calculated as Q1/Q3 ∓ 1.5 * IQR (interquartile range). Each point represents the value relative to a single gene.
Removal of the cap stimulates mRNA degradation of lowly transcribed genes
The similar patterns of mRNA levels in ceg1-AA and xrn1∆ strains imply that decapping is required for 5′−3′ Xrn1-dependent regulation of mRNA levels. Non-capped mRNAs may not be immediately subjected to efficient degradation upon Ceg1 depletion as they are not direct substrates for the 5′−3′ exonucleases and require modifications of the 5′ ends by other enzymes44, 45. Thus, we anticipated that even lowly expressed non-capped mRNAs with a 5′ end suitable for the 5′−3′ exonucleases would be efficiently removed and would not accumulate as observed following Ceg1 nuclear depletion. To test this hypothesis, we introduced the self-cleaving hepatitis delta virus (HDV) ribozyme sequence into the 5′ UTR of the FMP27 gene (RZ-fmp27) (Fig. 7a) in the ceg1-AA strain. The internal self-cleavage in the ribozyme sequence generated a non-capped transcript RZ-fmp27 with a 5′-OH end which is a suitable substrate for 5′−3′ exonucleases44,45. As a control, we used the catalytically inactive ribozyme mutant harbouring the substitution C76:U (C76U-fmp27) preventing the cleavage50,51. Both forms were expressed from the ADH1 promoter which increased C76U-fmp27 levels ~6 times compared to the WT FMP27 level (Fig. 7b) however, this expression was still magnitudes lower than ADH1 levels (Fig. 7c), retaining C76U-fmp27 in the range of lowly expressed genes. Our analysis showed that lowly expressed FMP27 mRNA did not change when synthesised as non-capped RNA in ceg1-AA (Fig. 4b). Similarly, C76U-fmp27 level was not affected by Ceg1 nuclear depletion (Fig. 7d). However, the functional ribozyme generating a non-capped transcript decreased the levels of RZ-fmp27 by 67% compared to the catalytically inactive C76U-fmp27 on DMSO (WT condition) and by 70% during Ceg1 nuclear depletion (Fig. 7d). This indicates that RZ-fmp27 mRNA decapped by the self-cleaving ribozyme, which produced a 5′ end suitable for 5′−3′ exonucleases, was efficiently degraded. This suggests that the generation of the correct substrate was required for the 5′ quality control as previously reported46.
a The experimental approach for the generation of the self-cleaving RZ-fmp27 transcript. Comparison of C76U-fmp27 mRNA levels with WT FMP27 (b) and ADH1 (c) mRNAs. d mRNA levels of RZ-fmp27 and C76U-fmp27 in ceg1-AA growing on Rap or DMSO. b, d The error bars show the standard deviation of three independent RT-qPCR experiments.
Discussion
Capping is the first co-transcriptional RNA modification imposed on mRNAs. The m7G cap is essential for the functionality of the mRNA and therefore, mutations affecting the cap formation are non-viable. Here, we performed a global analysis of the cap defective transcriptome. We show that although the decrease of mRNA levels was a dominant effect caused by the nuclear depletion of the capping enzymes, many mRNAs increased when not capped. This seems to contrast the previous studies reporting that selected RNAs with defective caps (e.g., lacking cap or with non-methylated caps) are rapidly degraded by 5′−3′ exonucleases1, 14,20. These reports indicated the role of Rai1 and Rat1 in degradation of capped but not methylated mRNAs14 and showed that Rat1 mutation initially slowed down degradation of non-capped FMP27 and GAL1 mRNAs but overall did not rescue their levels in ceg1-63 mutant20. Our genome-wide data show a clear dependence between the expression strength and the degradation of uncapped transcripts. The nuclear depletion of Ceg1 resulted in the reduction of highly expressed mRNAs and the accumulation of lowly expressed and more stable mRNAs. We observed the same pattern in cells lacking the 5′−3′ exonuclease Xrn1 which is one the major enzymes degrading mRNA in the cytoplasm. Consistently, we did not detect any significant changes in RNA levels in the xrn1Δ ceg1-AA strain when compared to the single ceg1-AA mutant. Our data indicate that the presence of cap is crucial to initiate 5′−3′ exonucleolytic degradation and is required for the Xrn1-dependent control and buffering of the global mRNA levels22. Indeed, a subunit of the decapping complex Dcp1-Dcp2, Dcp1 interacts with Xrn1, and the cap removal is the first step triggering mRNA degradation21,52. Thus, mRNA synthesised as non-capped transcripts may not be efficiently degraded by 5′−3′ exonucleases like Xrn1 or Rat1. In such a scenario, the highly expressed non-capped RNAs decreases significantly as they may be preferentially degraded by the 3′−5′ degrading machinery involving deadenylases and the exosome complex40. This in turn, may increase the relative concentration of lowly transcribed mRNAs in the overall RNA fraction since their degradation may rely mainly on 5′−3′ pathway40 (Fig. 8). We speculate that the binding of the capping enzymes to the transcriptional machinery or/and the initiation of m7G cap synthesis, is required to activate the cap quality control pathway. Such a condition would allow for the detection and removal of mRNA with defective caps15 and also aid the production of other essential non-capped but stable RNA classes such as ribosomal RNA (rRNA) synthesised by Pol I7,53 whose processing and termination employs Rat154,55. Indeed, the capping process plays a role in marking transcripts as mRNAs. For example, yeast box C/D snoRNAs, a class of Pol II transcripts which do not possess the m7G cap as mature species, are subjected to mRNA processing pathways if the caps are not co-transcriptionally removed from their precursors56.
The m7G cap contributes but may not be essential for the protection from exonucleolytic 5′−3′ degradation, as the synthesised 5′ triphosphate non-capped mRNAs are not direct substrates of the 5′−3′ exonucleases44, 45. Indeed, the generation of the 5′ end suitable for 5′−3′ exonucleases by the insertion of the self-cleaving HDV ribozyme into the 5′ UTR of FMP27 resulted in enhanced degradation of this lowly transcribed mRNA in the WT strain, confirming that the formation of a correct substrate is crucial for the 5′−3′ degradation machinery. Similarly, Rat1/Xrn1-dependent 5′−3′ processing of yeast snoRNAs depends on capping removal by decapping enzymes or endonuclease Rnt157.
Finally, we did not observe a significant increase in premature transcription termination upon depletion of the capping enzymes. This is consistent with our data showing that Rat1 and Rai1 inactivation did not restore the levels of non-capped mRNAs, but in contrasts with previous models suggesting that such RNAs are degraded co-transcriptionally by Rat1 eliciting transcription termination16,17,18. Our ChIP analysis revealed that only a small amount of Pol II tested using 8WG16 antibody was depleted from the chromatin in ceg1-AA. This antibody recognises unphosphorylated CTD and therefore the precipitated fraction may be enriched for hypophosphorylated Pol II. Such Pol II may not efficiently transit to the elongation phase, pause or transcribe at a low speed58,59,60. All these conditions facilitate transcription termination, which may be reflected by the lowered levels of Pol II observed in the ChIP analyses employing 8WG16 antibody. This effect was not detected when the distribution of Pol II was measured using antibodies recognising actively transcribing complexes. We speculate that for average length noncapped mRNAs (1.4 kb), the opportunity window is too narrow for Rai1-Rat1 to be recruited to the transcribing complex, modify the 5′ end and initiate co-transcriptional degradation and so to trigger transcription termination. Such premature transcription termination is observed when nascent RNAs are decapped which enforces Pol II pausing facilitating Rat1-dependent termination18,19,61,62.
Overall, our work reveals the complexity of the 5′ cap functions in cellular mRNA metabolism. The action of the capping enzyme during transcription and then the presence of the cap structure on the mature mRNA as well as the process of its removal generating an mRNA substrate for 5′−3′ exonucleases may all be crucial for the degradation of mRNA transcribed on low levels in the cell. Overall, cap may play an essential role in marking mRNA molecules and cooperating with Xrn1-dependent degradation and regulation of the cellular mRNA levels.
Methods
Oligonucleotides used in the study
Oligonucleotides used for strain construction, RT-qPCRs and ChIP analyses are listed in Supplementary Data 3.
Yeast strain and media
Strains: HHY168 (MATalpha tor1-1 fpr1::NAT RPL13AxFKB12::TRP), Euroscarf); ceg1-AA (HHY168 CEG1-FRB::HIS), this work; ceg1-GFP-AA (HHY168 CEG1-GFP-FRB::HIS), this work; cet1-AA (HHY168 CET1-FRB::HIS), this work; abd1-AA (HHY168 ABD1-FRB::HIS), this work; cbp20-AA (HHY168 CBP20-FRB::HIS); this work; cbp20-GFP-AA (HHY168 CBP20-GFP-FRB::KAN), this work; cbp80-AA (HHY168 CBP80-FRB::HIS), this work; cbp80-GFP-AA (HHY168 CBP80-GFP-FRB::KAN), this work; ceg1-AA/xrn1Δ (HHY168 CEG1-FRB::HIS KAN::XRN1), this work; ceg1-AA/rrp6Δ (HHY168 CEG1-FRB::HIS KAN::RRP6), this work; ceg1-63 (MATa ceg1Δ::HIS3 PGAL1-YLR454::URA3 ura3Δ [pRS315-ceg1-63])20; ceg1-63/rat1-1 (MATa rat1-1 ceg1Δ::HIS3 PGAL1-YLR454::URA3 ura3Δ [pRS315-ceg1-63])20; fmp27-C76U (HHY168 CEG1-FRB::HIS URA3::RZc76u::YLR454), this work; fmp27-RZ (HHY168 CEG1-FRB::HIS URA3::RZ::YLR454), this work; ceg1-AA/rai1Δ (HHY168 CEG1-FRB::HIS KAN::RAI1), this work.
Yeast strains were grown at 30 °C on YPD (1% yeast extract, 2% peptone, 2% dextrose/glucose) media enriched in DMSO or rapamycin to the final concentration of 1 µg/mL where indicated. For 6-AU treatment, the yeast cells were grown in minimal media (yeast nitrogen base without amino acids) with synthetic dropout ura-. For solid media, agar was added to the final concentration of 2%.
Growth spot assay
Yeast cells were grown to OD600 = 0.4. Serial dilutions 1:25 were performed and 5 µL of each dilution was spotted onto YPD (2% agar) plates.
Yeast cell transformation
Cells were cultured to OD600 = 1 and pelleted by centrifugation for 5 min at 2400 × g at room temperature (RT). The pellet was resuspended in sterile 10 mM Tris-HCl pH 7.5 or water and centrifuged again as before. The pellet was resuspended in filtered LiT (10 mM Tris-HCl pH 7.5;100 mM Lithium acetate), 1 M DTT and incubated at RT for 40 min with gentle shaking. After incubation the cells were pelleted again (as before) and resuspended in LiT and 1 M DTT. The suspension was added with LiT; and dsDNA (10 mg/mL); transforming DNA (0.1 to 1 µg or more) and incubated at RT for 10 min. Next, PEG solution (1:1 PEG4000:LiT) was added to the suspension and incubated at RT for 10 min. DMSO was then added, and the suspension incubated at 42 °C for 15 min. After the incubation, the suspension was pelleted and resuspended in 1 mL of YPD (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose/dextrose). Then, the suspension was incubated at 30 °C for 1 h, pelleted with 10 s spin, max speed and inoculated on selective plates.
RNA extraction
RNA was purified by phenol/chloroform method. The yeasts were cultured to the final concentration OD600 = 1, pelleted in 50 mL falcon tubes by centrifugation for 2 min at 1000 rpm at RT. The pellet was frozen at −80 °C before further manipulation. Next, the pellet was resuspended in 1 mL of ice-cold RNase-free water, moved to a 1.5 mL tube, centrifuged for 10 s at 4 °C max speed. Then the supernatant (water) was removed, and the pellet was resuspended in 400 µL of filtered AE buffer (50 mM Sodium acetate pH5.3; 10 mM EDTA), 40 µL of 10% Sodium dodecyl sulphate (SDS) and 400 µL of Acid Phenol (pH 4). The suspension was vortexed for 20 s and incubated at 65 °C for 10 min, then incubated for further 10 min at −80 °C. The defrosted sample was then centrifuged for 5 min, 13,000 rpm at RT. 400 µL of 1:1 phenol:chloroform solution were added to the supernatant and vortexed for 30 s. Then the sample was centrifuged 10 min, 13,000 rpm at RT. The upper phase was transferred and mixed with 400 µL of Chloroform, vortexed and centrifuged for 5 min, 13,000 rpm at RT. The RNA was precipitated by adding to the upper phase 1 mL of 100% ethanol and 40 µL of 7.5 M Ammonium acetate. Left at −80 °C for 2 h then centrifuged for 20 min, 13,000 at 4 °C. The pellet was washed with 70% ethanol (in water) and resuspended in RNase-free water.
RT-qPCR
Reverse transcription was performed with Super Script III Reverse transcriptase (Thermo Fisher, 18080044) following manufacturer’s instruction using random hexamers (Thermo Fisher, N8080127) for cDNA synthesis. qPCR data were analysed with the ∆∆Ct method normalised to 25S rRNA and control condition. The qPCR source data are listed in Supplementary Data 2.
RNA sequencing
Before library preparation, rRNA depletion was performed on 5 µg of total yeast RNA using 200 pmol of 3′ biotinylated probes (IDT) designed in house following the RiboPOP method63. The libraries preparation was performed with Ultra II RNA Library Prep Kit for Illumina (NEB, E7770) and Multiplex Oligos for Illumina (NEB, E7335) according to manufacturer’s instructions. The RNA sequencing was performed from the Genomics Facility at the University of Birmingham on Illumina NEXTseq apparatus.
RNA-seq data analysis
The quality of the data was checked using FastQC v0.11.5 (Andrews S. (2010). FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc).
Reads were aligned to the yeast genome R64 (sacCer3) using HISAT2 v2.1.1 and sorted and indexed using SAMtools v1.15.164. The read count per gene was calculated with LiBiNorm v.2.565 with the following parameters count -u name-root -f --order=pos --minaqual=10 --mode=intersection-strict --idattr=gene_id --type=exon. Differential expression analysis was performed using DESeq2 package v 4.3.166
The GC content relative to the differentially expressed genes was calculated via EMBOSS infoseq (www.bioinformatics.nl/cgi-bin/emboss/infoseq). Increased (Ic) and decreased (Dc) genes were selected according to the results obtained from differential expression analysis performed via DESeq2 as previously described and their sequences interrogated for the distribution of GC content. The codon bias analysis was performed employing the cordon R package67; R package version 1.18.0, https://github.com/BioinfoHR/coRdon).
Cap immunoprecipitation
Total RNA from cells treated with DMSO or rapamycin was extracted and used for cap pull-down. 5 µg of total yeast RNA was spiked with 1 µg of total human RNA as immunoprecipitation efficiency control. Capped RNA species were immunoprecipitated using 5 µg of anti m7G cap antibody (H-20) as previously described20.
6-azauracil (6-AU) treatment
Overnight culture was prepared inoculating a single colony on minimal media as described above. The next day, the culture was diluted to OD600 = 0.2. The diluted culture was grown with agitation at 30 °C to OD600 = 1 and split in 4. Two cultures were added of 6-azauracil (6-AU) and the other two with ammonium hydroxide (NH4OH, control) at the final concentration of 50 µg/mL for 30 min. Next, DMSO or rapamycin (Rap) at the final concentration of 1 µg/mL were added according to the following scheme: 6-AU + DMSO, 6-AU+Rap, NH4OH + DMSO, NH4OH+Rap. 10 mL of each suspension were collected a time 0, 45, 90 and 120 min after the addition of DMSO or Rap and prepared for RNA purification.
Chromatin immunoprecipitation (ChIP)
The specific yeast strain was grown over-night to OD600 = 1. Then 37% formaldehyde was added to the media to final concentration 1% dropwise with gentle shaking for 15 min. Next, 15 mL of 2.5 M glycine was added for further 5 min keeping the shaking. The cells were pelleted by centrifugation at 3500 rpm for 3 min at 4 °C. The pellet was resuspended in 40 mL of ice-cold 1x PBS and centrifuged at 3500 rpm for 5 min at 4 °C, twice. The pellet obtained was then transferred and prepared for lysis. The pellet was resuspended in 1 mL of ice-cold FA1 (50 mM HEPES-KOH, pH7.5; 150 mM NaCl; 1 mM EDTA; 1% triton-x 100; 0.1% Sodium deoxycholate) added with proteinase inhibitor (Roche Mini-EDTA free) and about 400 µL zirconia beads. The cells were lysed in MagnaLyser (30 s 7000 rpm, 3 cycles with 5 min incubation on ice in between). The lysate was drained out of the beads by centrifugation 1000 rpm for 1 min at 4 °C and SDS was added to a final concentration of 0.05%. The lysate was sonicated as follows: 25 cycles 15 s ON, 15 s OFF on maximum power. Next, it was centrifuged for 20 min at 12,500 rpm at 4 °C. 200 µL of lysate was saved to check the fragmentation. SDS was added to the lysate with to final concentration of 1% and 0.05 mg of Proteinase K and incubated at 42 °C for 1 h. Next it was moved to 65 °C for 4 h. The DNA de-crosslinked was precipitated by Phenol/Chloroform, the contaminant RNA was removed by adding 2 µL of RNase A incubating for 1 h at 37 °C and checked on agarose gel. The rest of the lysate was centrifuged at max speed for 5 min at 4 °C. 150 µg of chromatin were used for immunoprecipitation. FA1 enriched with proteinase inhibitor was added to the DNA to a final volume of 700 µL. 20 µL of such mix was saved as input for the further ChIP analysis. 2 µg of the specific antibody was added to the sample and incubated on rotation over-night at 4 °C.
15 µL of protein A (Thermo Fisher, 10002D) and 15 µL of protein G-coated beads (Thermo Fisher, 10004D) were washed with 1 mL of FA1 and resuspended in 100 µL of FA1. The beads were added to the sample and incubated by rotation for 1 h at 4 °C. After incubation the beads were washed six times with FA1, 1 time with FA2 (50 mM HEPES-KOH, pH 7.5; 500 mM NaCl; 1 mM EDTA; 1% Triton-X 100; 0.1% Sodium deoxycholate), one time with FA3 (20 mM Tris pH 8.0; 250 mM LiCl; 0.5% NP-40; 0.5% Sodium deoxycholate; 1 mM EDTA) and TE buffer pH 8 (100 mM Tris-HCl; 10 mM EDTA). The beads were then resuspended in 195 µL of Elution buffer (50 mM Tris pH 7.5; 10 mM EDTA; 1% SDS) and incubated for 10 min at 65 °C and 0.05 mg of Proteinase K added. The Chromatin was then de-crosslinked by incubating the sample at 42 °C for 1 h and moved at 65 °C for 4 h. The DNA was finally purified using Quiagen cleanup (28104) kit following the manufacturer’s instruction. The ChIP-qPCR source data are listed in Supplementary Data 2.
Microscopy of GFP-tagged strains
Fixed cell microscopy was performed on yeast cells grown to late exponential phase in Synthetic Complete media with 2% glucose (SCD). Cells were treated with DMSO or rapamycin to a final concentration of 1 µg/mL. 1 µL DAPI solution (2.5 mg/mL) was added to 1 mL treated yeast cells and vortexed gently before fixation in 100 µL 4% paraformaldehyde solution at room temperature for 15 min. 900 µL potassium phosphate/sorbitol (100 mM potassium phosphate pH 7.5, 1.2 M sorbitol) was added and cells centrifuged at top speed for 1 min, supernatant removed, and cells resuspended in 100 µL potassium sorbitol solution. 3 µL cells were mounted on 0.01% poly-L-lysine coated slides. Images were acquired using a Leica DM5500 fluorescent microscope. For quantification of the ratio between the mean signal intensity of the cytoplasm and nucleus, outlines were manually drawn using Image J for the whole cell and nucleus and the areas and mean signal intensities determined for each. The mean signal intensity of the cytoplasm was determined by calculating the total signal intensities, corrected against the background signal, and areas of the whole cell and the nucleus and subtracting that of the nucleus from the cytoplasm followed by calculation of the ratio between mean cytoplasmic signal versus mean nuclear signal. Statistical significance was determined through one way ANOVA.
Statistics and reproducibility
The results presented depict the output of independent experiments (n = 2–4). For each biological replicate shown, cells were grown, treated, and processed independently to minimise bias and validate reproducibility. The average values are shown, and the statistics performed via GraphPad Prism Version 10.0.2 represent the standard deviation resulting from the replicates analysed. For qPCR analysis where n = 2, only the individual points are shown. Statistical significance was calculated via t-test for the comparison between two groups and ANOVA for comparison of >2 groups (ns = P > 0.05; * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001; **** = P ≤ 0.0001). RNA-sequencing analyses were performed in bash with code generated in the lab and shown in the methods. The analysis and the statistics were performed via RStudio Version 2022.02.1 + 461.pro1 and the DESeq2 package v 4.3.1. The statistical significance was evaluated via padj method.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data for the ceg1-AA strains upon 45 min of treatment with DMSO or rapamycin are available in Gene Expression Omnibus (GEO) database with the accession number GSE213942. Source data for all graphs and charts in the main figures can be found in Supplementary Data 1 (Differentially expressed genes) and Supplementary Data 2 (qPCR values and microscopy quantifications). All other data are available from the corresponding author upon reasonable request.
References
Schwer, B., Mao, X. & Shuman, S. Accelerated mRNA decay in conditional mutants of yeast mRNA capping enzyme. Nucleic Acids Res. 26, 2050–2057 (1998).
Furuichi, Y. Discovery of m(7)G-cap in eukaryotic mRNAs. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 91, 394–409 (2015).
Izaurralde, E. et al. A cap-binding protein complex mediating U snRNA export. Nature 376, 709–712 (1995).
Izaurralde, E. et al. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell 78, 657–668 (1994).
Batool, A., Aashaq, S. & Andrabi, K. I. Eukaryotic initiation factor 4E (eIF4E): a recap of the cap-binding protein. J. Cell Biochem. 120, 14201–14212 (2019).
Decroly, E., Ferron, F., Lescar, J. & Canard, B. Conventional and unconventional mechanisms for capping viral mRNA. Nat. Rev. Microbiol. 10, 51–65 (2011).
Ramanathan, A., Robb, G. B. & Chan, S. H. mRNA capping: biological functions and applications. Nucleic Acids Res. 44, 7511–7526 (2016).
Varshney, D. et al. mRNA cap methyltransferase, RNMT-RAM, promotes RNA Pol II-dependent transcription. Cell Rep. 23, 1530–1542 (2018).
Schroeder, S. C., Schwer, B., Shuman, S. & Bentley, D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev. 14, 2435–2440 (2000).
Hausmann, S., Pei, Y. & Shuman, S. Homodimeric quaternary structure is required for the in vivo function and thermal stability of Saccharomyces cerevisiae and Schizosaccharomyces pombe RNA triphosphatases. J. Biol. Chem. 278, 30487–30496 (2003).
Martinez-Rucobo, F. W. et al. Molecular basis of transcription-coupled Pre-mRNA capping. Mol. Cell 58, 1079–1089 (2015).
Lidschreiber, M., Leike, K. & Cramer, P. Cap completion and C-terminal repeat domain kinase recruitment underlie the initiation-elongation transition of RNA polymerase II. Mol. Cell Biol. 33, 3805–3816 (2013).
Shatkin, A. J. & Manley, J. L. The ends of the affair: capping and polyadenylation. Nat. Struct. Biol. 7, 838–842 (2000).
Jiao, X. et al. Identification of a quality-control mechanism for mRNA 5′-end capping. Nature 467, 608–611 (2010).
Klama, S. et al. A guard protein-mediated quality control mechanism monitors 5′-capping of pre-mRNAs. Nucleic Acids Res. 50, 11301–11314 (2022).
Kim, M. et al. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432, 517–522 (2004).
West, S., Gromak, N. & Proudfoot, N. J. Human 5′ –> 3′ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature 432, 522–525 (2004).
Eaton, J. D., Francis, L., Davidson, L. & West, S. A unified allosteric/torpedo mechanism for transcriptional termination on human protein-coding genes. Genes Dev. 34, 132–145 (2020).
Cortazar, M. A. et al. Control of RNA Pol II speed by PNUTS-PP1 and Spt5 dephosphorylation facilitates termination by a “Sitting Duck Torpedo” mechanism. Mol. Cell 76, 896–908 e894 (2019).
Jimeno-Gonzalez, S., Haaning, L. L., Malagon, F. & Jensen, T. H. The yeast 5′−3′ exonuclease Rat1p functions during transcription elongation by RNA polymerase II. Mol. Cell 37, 580–587 (2010).
Braun, J. E. et al. A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat. Struct. Mol. Biol. 19, 1324–1331 (2012).
Sun, M. et al. Global analysis of eukaryotic mRNA degradation reveals Xrn1-dependent buffering of transcript levels. Mol. Cell 52, 52–62 (2013).
Haruki, H., Nishikawa, J. & Laemmli, U. K. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol. Cell 31, 925–932 (2008).
Marz, A. M., Fabian, A. K., Kozany, C., Bracher, A. & Hausch, F. Large FK506-binding proteins shape the pharmacology of rapamycin. Mol. Cell Biol. 33, 1357–1367 (2013).
Tsukamoto, T. et al. Isolation and characterization of the yeast mRNA capping enzyme beta subunit gene encoding RNA 5′-triphosphatase, which is essential for cell viability. Biochem. Biophys. Res. Commun. 239, 116–122 (1997).
Chu, C. & Shatkin, A. J. Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes. Mol. Cell Biol. 28, 5829–5836 (2008).
Mao, X., Schwer, B. & Shuman, S. Mutational analysis of the Saccharomyces cerevisiae ABD1 gene: cap methyltransferase activity is essential for cell growth. Mol. Cell Biol. 16, 475–480 (1996).
Pelechano, V., Wei, W. & Steinmetz, L. M. Extensive transcriptional heterogeneity revealed by isoform profiling. Nature 497, 127–131 (2013).
Geisberg, J. V., Moqtaderi, Z., Fan, X., Ozsolak, F. & Struhl, K. Global analysis of mRNA isoform half-lives reveals stabilizing and destabilizing elements in yeast. Cell 156, 812–824 (2014).
Chan, L. Y., Mugler, C. F., Heinrich, S., Vallotton, P. & Weis, K. Non-invasive measurement of mRNA decay reveals translation initiation as the major determinant of mRNA stability. Elife 7, https://doi.org/10.7554/eLife.32536 (2018).
Wyers, F. et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737 (2005).
Marquardt, S., Hazelbaker, D. Z. & Buratowski, S. Distinct RNA degradation pathways and 3′ extensions of yeast non-coding RNA species. Transcription 2, 145–154 (2011).
Xu, Z. et al. Bidirectional promoters generate pervasive transcription in yeast. Nature 457, 1033–1037 (2009).
Exinger, F. & Lacroute, F. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr. Genet. 22, 9–11 (1992).
Mason, P. B. & Struhl, K. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol. Cell 17, 831–840 (2005).
Reines, D. Use of RNA yeast polymerase II mutants in studying transcription elongation. Methods Enzymol. 371, 284–292 (2003).
Kudla, G., Lipinski, L., Caffin, F., Helwak, A. & Zylicz, M. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 4, e180 (2006).
Sharp, P. M., Tuohy, T. M. & Mosurski, K. R. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 14, 5125–5143 (1986).
Courel, M. et al. GC content shapes mRNA storage and decay in human cells. Elife 8, https://doi.org/10.7554/eLife.49708 (2019).
Sohrabi-Jahromi, S. et al. Transcriptome maps of general eukaryotic RNA degradation factors. Elife 8, https://doi.org/10.7554/eLife.47040 (2019).
Urrutia, A. O. & Hurst, L. D. The signature of selection mediated by expression on human genes. Genome Res. 13, 2260–2264 (2003).
Holtz-Heppelmann, C. J., Algeciras, A., Badley, A. D. & Paya, C. V. Transcriptional regulation of the human FasL promoter-enhancer region. J. Biol. Chem. 273, 4416–4423 (1998).
Thewes, S. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot. Cell 13, 694–705 (2014).
Doamekpor, S. K., Gozdek, A., Kwasnik, A., Kufel, J. & Tong, L. A novel 5′-hydroxyl dinucleotide hydrolase activity for the DXO/Rai1 family of enzymes. Nucleic Acids Res. 48, 349–358 (2020).
Jinek, M., Coyle, S. M. & Doudna, J. A. Coupled 5′ nucleotide recognition and processivity in Xrn1-mediated mRNA decay. Mol. Cell 41, 600–608 (2011).
Bresson, S. & Tollervey, D. Tailing off: PABP and CNOT generate cycles of mRNA deadenylation. Mol. Cell 70, 987–988 (2018).
Xiang, S. et al. Structure and function of the 5′–>3′ exoribonuclease Rat1 and its activating partner Rai1. Nature 458, 784–788 (2009).
Kachaev, Z. M., Lebedeva, L. A., Kozlov, E. N. & Shidlovskii, Y. V. Interplay of mRNA capping and transcription machineries. Biosci. Rep. 40, https://doi.org/10.1042/BSR20192825 (2020).
Hurowitz, E. H. & Brown, P. O. Genome-wide analysis of mRNA lengths in Saccharomyces cerevisiae. Genome Biol. 5, R2 (2003).
Bird, G., Fong, N., Gatlin, J. C., Farabaugh, S. & Bentley, D. L. Ribozyme cleavage reveals connections between mRNA release from the site of transcription and pre-mRNA processing. Mol. Cell 20, 747–758 (2005).
Perrotta, A. T., Shih, I. & Been, M. D. Imidazole rescue of a cytosine mutation in a self-cleaving ribozyme. Science 286, 123–126 (1999).
Schoenberg, D. R. & Maquat, L. E. Regulation of cytoplasmic mRNA decay. Nat. Rev. Genet. 13, 246–259 (2012).
Akiyama, B. M., Eiler, D. & Kieft, J. S. Structured RNAs that evade or confound exonucleases: function follows form. Curr. Opin. Struct. Biol. 36, 40–47 (2016).
El Hage, A., Koper, M., Kufel, J. & Tollervey, D. Efficient termination of transcription by RNA polymerase I requires the 5′ exonuclease Rat1 in yeast. Genes Dev. 22, 1069–1081 (2008).
Granneman, S., Petfalski, E. & Tollervey, D. A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease. EMBO J. 30, 4006–4019 (2011).
Grzechnik, P. et al. Nuclear fate of yeast snoRNA is determined by co-transcriptional Rnt1 cleavage. Nat. Commun. 9, 1783 (2018).
Kufel, J. & Grzechnik, P. Small nucleolar RNAs tell a different tale. Trends Genet. 35, 104–117 (2019).
Bartkowiak, B. & Greenleaf, A. L. Phosphorylation of RNAPII: To P-TEFb or not to P-TEFb? Transcription 2, 115–119 (2011).
Harlen, K. M. & Churchman, L. S. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat. Rev. Mol. Cell Biol. 18, 263–273 (2017).
Harlen, K. M. et al. Comprehensive RNA polymerase II interactomes reveal distinct and varied roles for each phospho-CTD residue. Cell Rep. 15, 2147–2158 (2016).
Brannan, K. et al. mRNA decapping factors and the exonuclease Xrn2 function in widespread premature termination of RNA polymerase II transcription. Mol. Cell 46, 311–324 (2012).
Eaton, J. D. & West, S. Termination of transcription by RNA polymerase II: BOOM! Trends Genet. 36, 664–675 (2020).
Thompson, M. K., Kiourlappou, M. & Davis, I. Ribo-Pop: simple, cost-effective, and widely applicable ribosomal RNA depletion. RNA 26, 1731–1742 (2020).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Dyer, N. P., Shahrezaei, V. & Hebenstreit, D. LiBiNorm: an htseq-count analogue with improved normalisation of Smart-seq2 data and library preparation diagnostics. PeerJ 7, e6222 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Elek A, K. M., Vlahovicek K. https://github.com/BioinfoHR/coRdon (2023).
Acknowledgements
This work was funded by Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (218537/Z/19/Z). O.Z. was supported by Midlands Doctoral Training Partnership by a studentship to O.Z. funded by BBSRC (grant number: BB/M01116X/1). D.H. is funded by EPSRC (grant EP/T002794/1). M.E. and M.A. are funded by BBSRC (BB/V015109/1). We thank Genomics Birmingham at the University of Birmingham for assistance with NGS.
Author information
Authors and Affiliations
Contributions
O.Z. performed most of the experiments (excluding approaches listed below), bioinformatics and data analyses. M.E. and M.A. performed microscopy, K.W. performed m7G cap immunoprecipitations and ribozyme analyses. D.H. supported bioinformatics. R.N.-M. supported data analysis. P.G. and O.Z. designed experimental approaches and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Per Sunnerhagen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Gene Chong, Christina Karlsson Rosenthal, and David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zanin, O., Eastham, M., Winczura, K. et al. Ceg1 depletion reveals mechanisms governing degradation of non-capped RNAs in Saccharomyces cerevisiae. Commun Biol 6, 1112 (2023). https://doi.org/10.1038/s42003-023-05495-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05495-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.