Abstract
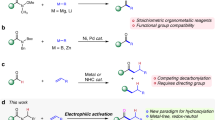
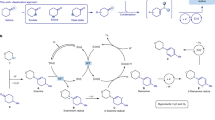
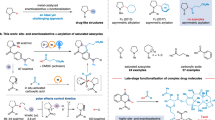
The introduction of alkylamines onto heteroaromatics is integral to the preparation of high-value molecules. Typical methods rely on heteroaromatic pre-functionalization by halogenation or nitration, followed by metal-catalysed cross-coupling or multi-step manipulation of the nitrogen functionality. This results in often unselective or low-yielding synthetic routes. Here we show an alternative approach in which saturated heterocyclic ketones are used as aryl surrogates for desaturative coupling with amines. The process operates under mild photochemical conditions, is compatible with complex amines and delivers both electron-poor and -rich heteroaromatics that are difficult to access by other methods. As ketones are readily decorated by carbonyl chemistry, this retrosynthetic tactic escapes the rules and limitations of aromatic reactivity and metal-catalysed cross-couplings. Our process uses enamine formation to create the key carbon–nitrogen bond, followed by two rounds of photoredox oxidation and cobalt-catalysed desaturation. The two desaturation steps are distinct, as the cobaloxime first acts as a hydrogen atom abstractor and then an oxidant.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information or from the authors upon reasonable request.
References
Bariwal, J. & Van Der Eycken, E. C–N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 42, 9283 (2013).
Hili, R. & Yudin, A. K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2, 284–287 (2006).
Mann, A. in Amino Group Chemistry: From Synthesis to the Life Sciences (ed. Ricci, A.) Ch. 6 (Wiley, 2008).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Katritzky, A. R., Ramsden, C. A., Joule, J. A. & Zhdankin, V. V. in Handbook of Heterocyclic Chemistry (eds Zhdankin, V. V. et al.) 3rd edn, Vol. 2 (Elsevier, 2010).
Fier, P. S., Kim, S. & Cohen, R. D. A multifunctional reagent designed for the site-selective amination of pyridines. J. Am. Chem. Soc. 142, 8614–8618 (2020).
Pang, J. H., Kaga, A., Roediger, S., Lin, M. H. & Chiba, S. Revisiting the chichibabin reaction: C2 amination of pyridines with a NaH−iodide composite. Asian J. Org. Chem. 8, 1058–1060 (2019).
Balkenhohl, M., Heinz, B., Abegg, T. & Knochel, P. Amination of phosphorodiamidate-substituted pyridines and related N-heterocycles with magnesium amides. Org. Lett. 20, 8057–8060 (2018).
Hendrick, C. E., Bitting, K. J., Cho, S. & Wang, Q. Site-selective copper-catalyzed amination and azidation of arenes and heteroarenes via deprotonative zincation. J. Am. Chem. Soc. 139, 11622–11628 (2017).
Pang, J. H., Kaga, A. & Chiba, S. Nucleophilic amination of methoxypyridines by a sodium hydride–iodide composite. Chem. Commun. 54, 10324–10327 (2018).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Creutz, S. E., Lotito, K. J., Fu, G. C. & Peters, J. C. Photoinduced Ullmann C–N coupling: demonstrating the viability of a radical pathway. Science 338, 647–651, (2012).
Roy, S., Paul, B., Mukherjee, A., Kundu, B. & Talukdar, A. Copper-catalyzed selective C–N bond formation with 2-amino, 2-hydroxy and 2-bromo-5-halopyridine. RSC Adv. 7, 44366–44370 (2017).
Corcoran, E. B. et al. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 353, 279–283 (2016).
Li, C. et al. Electrochemically enabled, nickel-catalyzed amination. Angew. Chem. Int. Ed. 56, 13088–13093 (2017).
Boyle, B. T., Levy, J. N., de Lescure, L., Paton, R. S. & McNally, A. Halogenation of the 3-position of pyridines through Zincke imine intermediates. Science 378, 773–779 (2022).
Cao, H., Cheng, Q. & Studer, A. Radical and ionic meta-C–H functionalization of pyridines, quinolines, and isoquinolines. Science 378, 779–785 (2022).
Charles, M. D., Schultz, P. & Buchwald, S. L. Efficient Pd-catalyzed amination of heteroaryl halides. Org. Lett. 7, 3965–3968, (2005).
Reichert, E. C., Feng, K., Sather, A. C. & Buchwald, S. L. Pd-catalyzed amination of base-sensitive five-membered heteroaryl halides with aliphatic amines. J. Am. Chem. Soc. 145, 3323–3329 (2023).
Arrechea, P. L. & Buchwald, S. L. Biaryl phosphine based Pd(II) amido complexes: the effect of ligand structure on reductive elimination. J. Am. Chem. Soc. 138, 12486–12493, (2016).
Hooper, M. W. & Hartwig, J. F. Understanding the coupling of heteroaromatic substrates: synthesis, structures, and reductive eliminations of heteroarylpalladium amido complexes. Organometallics 22, 3394–3403 (2003).
Sather, A. C. & Martinot, T. A. Data-rich experimentation enables palladium-catalyzed couplings of piperidines and five-membered (hetero)aromatic electrophiles. Org. Process Res. Dev. 23, 1725–1739 (2019).
Caldora, H. P., Zhang, Z., Tilby, M. J., Turner, O. & Leonori, D. Dual photochemical H‐atom transfer and cobalt catalysis for the desaturative synthesis of phenols from cyclohexanones. Angew. Chem. Int. Ed. 62, e202301656 (2023).
Deng, K., Huang, H. & Deng, G.-J. Recent advances in the transition metal-free oxidative dehydrogenative aromatization of cyclohexanones. Org. Biomolecular Chem. 19, 6380–6391 (2021).
Ichitsuka, T. et al. Stereoretentive N-arylation of amino acid esters with cyclohexanones utilizing a continuous‐flow system. Chem. Eur. J. 27, 10844–10848 (2021).
Kim, J. et al. Synthesis of N-aryl amines enabled by photocatalytic dehydrogenation. Chem. Sci. 12, 1915–1923 (2021).
Li, H., Yatabe, T., Takayama, S. & Yamaguchi, K. Heterogeneously catalyzed selective acceptorless dehydrogenative aromatization to primary anilines from ammonia via concerted catalysis and adsorption control. J. Am. Chem. Soc. Au 3, 1376–1384 (2023).
Qiu, Z., Zeng, H. & Li, C.-J. Coupling without coupling reactions: en route to developing phenols as sustainable coupling partners via dearomatization–rearomatization processes. Acc. Chem. Res. 53, 2395–2413 (2020).
Tao, S.-K. et al. Electrochemical cross-dehydrogenative aromatization protocol for the synthesis of aromatic amines. Org. Lett. 24, 1011–1016 (2022).
Dighe, S. U., Juliá, F., Luridiana, A., Douglas, J. J. & Leonori, D. A photochemical dehydrogenative strategy for aniline synthesis. Nature 584, 75–81 (2020).
Zhao, H., Caldora, H. P., Turner, O., Douglas, J. J. & Leonori, D. A desaturative approach for aromatic aldehyde synthesis via synergistic enamine, photoredox and cobalt triple catalysis. Angew. Chem. Int. Ed. 61, e202201870 (2022).
Afanasenko, A., Kavun, A., Thomas, D. & Li, C. J. A one‐pot approach for bio‐based arylamines via a combined photooxidative dearomatization–rearomatization strategy. Chem. Eur. J. 28, e202200309 (2022).
Huang, C.-Y., Li, J. & Li, C.-J. A cross-dehydrogenative C(sp3)−H heteroarylation via photo-induced catalytic chlorine radical generation. Nat. Commun. 12, 4010 (2021).
Li, J., Huang, C.-Y., Han, J.-T. & Li, C.-J. Development of a quinolinium/cobaloxime dual photocatalytic system for oxidative C–C cross-couplings via H2 release. ACS Catal. 11, 14148–14158 (2021).
He, K. H. et al. Acceptorless dehydrogenation of N‐heterocycles by merging visible‐light photoredox catalysis and cobalt catalysis. Angew. Chem. Int. Ed. 56, 3080–3084 (2017).
Jia, Z., Yang, Q., Zhang, L. & Luo, S. Photoredox mediated acceptorless dehydrogenative coupling of saturated N-heterocycles. ACS Catal. 9, 3589–3594 (2019).
West, J. G., Huang, D. & Sorensen, E. J. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun. 6, 10093 (2015).
Ritu et al. Photocatalyzed dehydrogenation of aliphatic N-heterocycles releasing dihydrogen. ACS Catal. 12, 10326–10332 (2022).
Bam, R., Pollatos, A. S., Moser, A. J. & West, J. G. Mild olefin formation via bio-inspired vitamin B12 photocatalysis. Chem. Sci. 12, 1736–1744 (2021).
West, J. G. & Kattamuri, P. V. Cooperative hydrogen atom transfer: from theory to applications. Synlett 32, 1179–1186 (2021).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Khadra, A., Mayer, S., Mitchell, D., Rodriguez, M. J. & Organ, M. G. A general protocol for the broad-spectrum cross-coupling of nonactivated sterically hindered 1° and 2° amines. Organometallics 36, 3573–3577 (2017).
Park, N. H., Vinogradova, E. V., Surry, D. S. & Buchwald, S. L. Design of new ligands for the palladium-catalyzed arylation of α-branched secondary amines. Angew. Chem. Int. Ed. 54, 8259–8262, (2015).
Barham, J. P., John, M. P. & Murphy, J. A. Contra-thermodynamic hydrogen atom abstraction in the selective C–H functionalization of trialkylamine N–CH3 groups. J. Am. Chem. Soc. 138, 15482–15487 (2016).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Guo, W. et al. Metal-free synthesis of N-aryl amides using organocatalytic ring-opening aminolysis of lactones. ChemSusChem 10, 1969–1975 (2017).
Bentabed-Ababsa, G. et al. Direct metalation of heteroaromatic esters and nitriles using a mixed lithium−cadmium base. Subsequent conversion to dipyridopyrimidinones. J. Org. Chem. 75, 839–847 (2010).
Levy, J. N., Alegre-Requena, J. V., Liu, R., Paton, R. S. & McNally, A. Selective halogenation of pyridines using designed phosphine reagents. J. Am. Chem. Soc. 142, 11295–11305 (2020).
Baker, S. I. et al. Enhanced reactivity for aromatic bromination via halogen bonding with lactic acid derivatives. J. Org. Chem. 87, 8492–8502 (2022).
Yu, Q., Hu, L. A., Wang, Y., Zheng, S. & Huang, J. Directed meta-selective bromination of arenes with ruthenium catalysts. Angew. Chem. Int. Ed. 54, 15284–15288 (2015).
Leclerc, G., Marciniak, G., Decker, N. & Schwartz, J. Cardiotonic agents. 1. Synthesis and structure–activity relationships in a new class of 3-, 4- and 5-pyridyl-2(1H)-quinolone derivatives. J. Med. Chem. 29, 2427–2432 (1986).
Bayliss, T. et al. Phosphoinositide 3-kinase inhibitor compounds and methods of use. US Patent WO/2008/070740 (2008).
Allen, L. J., Cabrera, P. J., Lee, M. & Sanford, M. S. N-Acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C–H amination of arenes and heteroarenes. J. Am. Chem. Soc. 136, 5607–5610, (2014).
Foo, K., Sella, E., Thomé, I., Eastgate, M. D. & Baran, P. S. A mild, ferrocene-catalyzed C–H imidation of (hetero)arenes. J. Am. Chem. Soc. 136, 5279–5282 (2014).
Kim, H., Kim, T., Lee, D. G., Roh, S. W. & Lee, C. Nitrogen-centered radical-mediated C–H imidation of arenes and heteroarenes via visible light induced photocatalysis. Chem. Commun. 50, 9273–9276 (2014).
Cao, H., Cheng, Q. & Studer, A. meta-Selective C−H functionalization of pyridines. Angew. Chem. Int. Ed. 62, e202302941 (2023).
Josephitis, C. M., Nguyen, H. M. H. & McNally, A. Late-stage C–H functionalization of azines. Chem. Rev. 123, 7655–7691 (2023).
Broggi, J., Clavier, H. & Nolan, S. P. N-Heterocyclic carbenes (NHCs) containing N–C-palladacycle complexes: synthesis and reactivity in aryl amination reactions. Organometallics 27, 5525–5531 (2008).
Ruiz-Castillo, P., Blackmond, D. G. & Buchwald, S. L. Rational ligand design for the arylation of hindered primary amines guided by reaction progress kinetic analysis. J. Am. Chem. Soc. 137, 3085–3092 (2015).
Shen, Q., Ogata, T. & Hartwig, J. F. Highly reactive, general and long-lived catalysts for palladium-catalyzed amination of heteroaryl and aryl chlorides, bromides, and iodides: scope and structure-activity relationships. J. Am. Chem. Soc. 130, 6586–6596 (2008).
Guthikonda, R. N. et al. Structure–activity relationships in the 2-arylcarbapenem series. Synthesis of 1-methyl-2-arylcarbapenems. J. Med. Chem. 30, 871–880 (1987).
Li, Y., Plesescu, M. & Prakash, S. R. Synthesis of C-14 and C-13, H-2-labeled IKK inhibitor: [14C] and [13C4,D3]-N-(6-chloro-7-methoxy-9H-pyrido[3,4-b]indol-8-yl)-2-methyl-3-pyridinecarboxamide. J. Label. Compd. Radiopharm. 49, 789–799 (2006).
Prabhath, M. R. R., Romanova, J., Curry, R. J., Silva, S. R. P. & Jarowski, P. D. The role of substituent effects in tuning metallophilic interactions and emission energy of bis-4-(2-pyridyl)-1,2,3-triazolatoplatinum(II) complexes. Angew. Chem. Int. Ed. 54, 7949–7953 (2015).
Benson, S. C., Li, J. H. & Snyder, J. K. Indole as a dienophile in inverse electron demand Diels–Alder reactions. 3. Intramolecular reactions with 1,2,4-triazines to access the canthine skeleton. J. Org. Chem. 57, 5285–5287 (1992).
Gruseck, U. & Heuschmann, M. The remarkable reactivity of 2-alkylidene-imidazolidines in inverse Diels-Alder reactions. Tetrahedron Lett. 28, 6027–6030 (1987).
Jalani, H. B. et al. Iodine-promoted one-pot synthesis of highly substituted 4-aminopyrroles and bis-4-aminopyrrole from aryl methyl ketones, arylamines, and enamines. Adv. Synth. Catal. 360, 4073–4079 (2018).
Kumari, C. & Goswami, A. Access to 5-substituted 3-aminofuran/thiophene-2-carboxylates from bifunctional alkynenitriles. Adv. Synth. Catal. 364, 2254–2259 (2022).
Lei, X., Li, L., He, Y.-P. & Tang, Y. Rhodium(II)-catalyzed formal [3+2] cycloaddition of N-sulfonyl-1,2,3-triazoles with isoxazoles: entry to polysubstituted 3-aminopyrroles. Org. Lett. 17, 5224–5227 (2015).
Li, K. & You, J. Cascade oxidative coupling/cyclization: a gateway to 3-amino polysubstituted five-membered heterocycles. J. Org. Chem. 81, 2327–2339 (2016).
Peng, J. et al. Synthesis of polysubstituted 3-amino pyrroles via palladium-catalyzed multicomponent reaction. J. Org. Chem. 82, 3581–3588 (2017).
Wang, Y., Lei, X. & Tang, Y. Rh(II)-catalyzed cycloadditions of 1-tosyl 1,2,3-triazoles with 2H-azirines: switchable reactivity of Rh-azavinylcarbene as [2C]- or aza-[3C]-synthon. Chem. Commun. 51, 4507–4510 (2015).
You, X. et al. Titanium-mediated cross-coupling reactions of 1,3-butadiynes with α-iminonitriles to 3-aminopyrroles: observation of an imino aza-Nazarov cyclization. Org. Chem. Front. 1, 940–946 (2014).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363, (2013).
Maillard, P. & Giannotti, C. Photolysis of alkylcobaloximes, methyl-salen, cobalamines and coenzyme B12 in protic solvents: an ESR and spin-trapping technique study. J. Organomet. Chem. 182, 225–237 (1979).
Schrauzer, G. N., Lee, L.-P. & Sibert, J. W. Alkylcobalamins and alkylcobaloximes. Electronic structure, spectra, and mechanism of photodealkylation. J. Am. Chem. Soc. 92, 2997–3005 (1970).
Sun, X., Chen, J. & Ritter, T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nat. Chem. 10, 1229–1233 (2018).
Dempsey, J. L., Brunschwig, B. S., Winkler, J. R. & Gray, H. B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 42, 1995–2004 (2009).
Elgrishi, N., Kurtz, D. A. & Dempsey, J. L. Reaction parameters influencing cobalt hydride formation kinetics: implications for benchmarking H2-evolution catalysts. J. Am. Chem. Soc. 139, 239–244 (2017).
Estes, D. P., Grills, D. C. & Norton, J. R. The reaction of cobaloximes with hydrogen: products and thermodynamics. J. Am. Chem. Soc. 136, 17362–17365 (2014).
Jiang, Y.-K. & Liu, J.-H. DFT studies of cobalt hydride intermediate on cobaloxime-catalyzed H2 evolution pathways. Int. J. Quantum Chem. 112, 2541–2546 (2012).
Lacy, D. C., Roberts, G. M. & Peters, J. C. The cobalt hydride that never was: revisiting Schrauzer’s “hydridocobaloxime”. J. Am. Chem. Soc. 137, 4860–4864 (2015).
Cartwright, K. C., Davies, A. M. & Tunge, J. A. Cobaloxime‐catalyzed hydrogen evolution in photoredox‐facilitated small‐molecule functionalization. Eur. J. Org. Chem. 2020, 1245–1258 (2020).
Basel, Y. & Hassner, A. Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols1. J. Org. Chem. 65, 6368–6380 (2000).
Fersht, A. R. & Jencks, W. P. Acetylpyridinium ion intermediate in pyridine-catalyzed hydrolysis and acyl transfer reactions of acetic anhydride. Observation, kinetics, structure–reactivity correlations, and effects of concentrated salt solutions. J. Am. Chem. Soc. 92, 5432–5442 (1970).
Acknowledgements
D.L. thanks the European Research Council for a research grant (101086901). H.P.C. thanks AstraZeneca for a PhD CASE Award. J.C. thanks the EU funding from an MSCA Postdoctoral Fellowship (101104383-DES-B-CAT). L.M.A. is a Ramón y Cajal fellow (ref. RYC2021-030994-I) and thanks MCIN/AEI and NextGenerationEU/PRTR for support and the KAUST Supercomputer Laboratory (KSL) for providing the computational resources (Shaheen II). We thank C. Vermeeren (RWTH Aachen University) for help with the purification of some of the products.
Author information
Authors and Affiliations
Contributions
A.R. and D.L. designed the project and directed the work. J.C., H.C. and E.M.d.T. performed all the synthetic and mechanistic experiments. L.M.A. performed the computational studies. All the authors analysed the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
Authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Julian West, Wen-Jing Xiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22 and Tables 1–20.
Supplementary Data 1
Coordinates (xyz) of the computational section.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Corpas, J., Caldora, H.P., Di Tommaso, E.M. et al. A general strategy for the amination of electron-rich and electron-poor heteroaromatics by desaturative catalysis. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01152-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01152-1