Abstract
Wastewater discharge to rivers is a controversial practice that compromises water quality, aquatic habitats and human health worldwide. Here we show how untreated wastewater laced with microplastics and raw sewage is routinely discharged into UK river flows that are too low to disperse the microplastics downstream. These ‘dry weather’ spills lead to acute microplastic contamination of river bed habitats. Many aquatic fauna feed in the benthic zone, the quality of which affects the entire riverine ecosystem. All microplastic types accumulate to high concentrations on the channel bed until flushed downstream by floods. These findings pose fundamental questions about the sustainable management of urban wastewater. Treating the wastewater would shut down the major source of microplastic fragments and microbeads in such rivers and prevent their transport to the ocean. Riverine microplastic transport is primarily partitioned between: (1) continuous transport at low concentrations of synthetic fibres from treated wastewater effluent; and (2) episodic flood-driven transport of the full microplastic assemblage entrained from contaminated channel beds. Focusing only on the buoyant non-flood microplastic load can produce highly unrepresentative assessments of riverine microplastic contamination. Climate warming and urban population growth will intensify the microplastic burden on many river ecosystems as summer baseflows decline and wastewater fluxes increase.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Rowley, K. H., Cucknell, A.-C., Smith, B. D., Clark, P. F. & Morritt, D. London’s river of plastic: high levels of microplastics in the Thames water column. Sci. Total Environ. 740, 140018 (2020).
Hurley, R. R., Woodward, J. C. & Rothwell, J. J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 11, 251–257 (2018).
Rochman, C. M. Microplastics research—from sink to source. Science 360, 28–29 (2018).
Siegfried, M., Koelmans, A. A., Besseling, E. & Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 127, 249–257 (2017).
Van Sebille, E. et al. A global inventory of small floating plastic debris. Environ. Res. Lett. 10, 124006 (2015).
Ross, P. S. et al. Pervasive distribution of polyester fibres in the Arctic Ocean is driven by Atlantic inputs. Nat. Commun. 12, 106 (2021).
Napper, I. E. et al. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 274, 116348 (2021).
Long, Z. et al. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 155, 255–265 (2019).
López-Rojo, N. et al. Microplastics have lethal and sublethal effects on stream invertebrates and affect stream ecosystem functioning. Environ. Pollut. 259, 113898 (2020).
Hurley, R. R., Woodward, J. C. & Rothwell, J. J. Ingestion of microplastics by freshwater tubifex worms. Environ. Sci. Technol. 51, 12844–12851 (2017).
Su, L. et al. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 234, 347–355 (2018).
Horton, A. A., Jürgens, M. D., Lahive, E., van Bodegom, P. M. & Vijver, M. G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 236, 188–194 (2018).
Smiroldo, G., Balestrieri, A., Pini, E. & Tremolada, P. Anthropogenically altered trophic webs: alien catfish and microplastics in the diet of Eurasian otters. Mammal. Res. 64, 165–174 (2019).
D’Souza, J. M., Windsor, F. M., Santillo, D. & Ormerod, S. J. Food web transfer of plastics to an apex riverine predator. Glob. Change Biol. 26, 3846–3857 (2020).
Mateos-Cárdenas, A. et al. Rapid fragmentation of microplastics by the freshwater amphipod Gammarus duebeni (Lillj.). Sci. Rep. 10, 12799 (2020).
Windsor, F. M. et al. A catchment‐scale perspective of plastic pollution. Glob. Change Biol. 25, 1207–1221 (2019).
Tang, Y. et al. Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species, Tegillarca granosa. Environ. Pollut. 265, 115115 (2020).
Woodward, J. C., Rothwell, J. J., Hurley, R. R., Li, J. & Ridley, M. Microplastics in rivers. Environ. Scientist. 29, 36–43 (2020).
Townsend, C. R. & Hildrew, A. G. Field experiments on the drifting, colonization and continuous redistribution of stream benthos. J. Anim. Ecol. 45, 759–772 (1976).
Comber, S. D. W., Gardner, M. J. & Ellor, B. Seasonal variation of contaminant concentrations in wastewater treatment works effluents and river waters. Environ. Technol. 41, 2716–2730 (2019).
River Tame at Portwood (69027) (UK National River Flow Archive, 2020); https://nrfa.ceh.ac.uk/data/station/info/69027.html
Upper Mersey Catchment Management Plan (National Rivers Authority, 1996).
Sutton, R. et al. Microplastic contamination in the San Francisco Bay, California, USA. Mar. Poll. Bull. 109, 230–235 (2016).
Gündoğdu, S., Çevik, C., Güzel, E. & Kilercioğlu, S. Microplastics in municipal wastewater treatment plants in Turkey: a comparison of the influent and secondary effluent concentrations. Environ. Monit. Assess. 190, 626 (2018).
Vermaire, J. C., Pomeroy, C., Herczegh, S. M., Haggart, O. & Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets (Ott.) 2, 301–314 (2017).
Lv, X. et al. Microplastics in a municipal wastewater treatment plant: fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 225, 579–586 (2019).
Murphy, F., Ewins, C., Carbonnier, F. & Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 50, 5800–5808 (2016).
Tibbetts, J., Krause, S., Lynch, I. & Sambrook Smith, G. H. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 10, 1597 (2018).
Horton, A. A., Svendsen, C., Williams, R. J., Spurgeon, D. J. & Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 114, 218–226 (2017).
Ockelford, A., Cundy, A. & Ebdon, J. E. Storm response of fluvial sedimentary microplastics. Sci. Rep. 10, 1865 (2020).
Blair, R. M., Waldron, S. & Gauchotte-Lindsay, C. Average daily flow of microplastics through a tertiary wastewater treatment plant over a ten-month period. Water Res. 163, 114909 (2019).
Curtis, E. J. C. & Harrington, D. W. The occurrence of sewage fungus in rivers in the United Kingdom. Water Res. 5, 281–290 (1971).
Fowles, A. E., Edgar, G. J., Hill, N., Stuart-Smith, R. D. & Kirkpatrick, J. B. An experimental assessment of impacts of pollution sources on sessile biota in a temperate urbanised estuary. Mar. Pollut. Bull. 133, 209–217 (2018).
Harvey, G. L. et al. Invasive crayfish as drivers of fine sediment dynamics in rivers: field and laboratory evidence. Earth Surf. Process. Landf. 39, 259–271 (2014).
Wessel, C. C., Lockridge, G. R., Battiste, D. & Cebrian, J. Abundance and characteristics of microplastics in beach sediments: Insights into microplastic accumulation in northern Gulf of Mexico estuaries. Mar. Pollut. Bull. 109, 178–183 (2016).
Kowalski, N., Reichardt, A. M. & Waniek, J. J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 109, 310–319 (2016).
Corcoran, P. L. Benthic plastic debris in marine and freshwater environments. Environ. Sci. Process Impacts 17, 1363–1369 (2015).
Woodall, L. C. et al. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 1, 140317 (2014).
Kane, I. A. et al. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 368, 1140–1145 (2020).
Laville, S. Water firms discharged raw sewage into English waters 400,000 times last year. The Guardian (31 March 2021); https://www.theguardian.com/environment/2021/mar/31/water-firms-discharged-raw-sewage-into-english-waters-400000-times-last-year
Hammond, P. et al. Detection of untreated sewage discharges to watercourses using machine learning. NPJ Clean Water 4, 18 (2021).
Acreman, M. C. & Ferguson, A. J. D. Environmental flows and the European Water Framework Directive. Freshw. Biol. 55, 32–48 (2010).
Qiao, R., Lu, K., Deng, Y., Ren, H. & Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 682, 128–137 (2019).
Sustainable Goal 6: Ensure Availability and Sustainable Management of Water and Sanitation for All (United Nations, 2015); https://sdgs.un.org/goals/goal6 .
Sadoff, C. W., Borgomeo, E. & Uhlenbrook, S. Rethinking water for SDG 6. Nat. Sustain. 3, 346–347 (2020).
Garner, G., Hannah, D. M. & Watts, G. Climate change and water in the UK: recent scientific evidence for past and future change. Prog. Phys. Geogr. Earth Environ. 41, 154–170 (2017).
Prudhomme, C., Young, A. & Watts, G. The drying up of Britain? A national estimate of changes in seasonal river flows from 11 regional climate model simulations. Hydrol. Process. 26, 1115–1118 (2012).
Miller, J. D. & Hutchins, M. The impacts of urbanisation and climate change on urban flooding and urban water quality: a review of the evidence concerning the United Kingdom. J. Hydrol. Reg. Stud. 12, 345–362 (2017).
Lambert, C. P. & Walling, D. E. Measurement of channel storage of suspended sediment in a gravel-bed river. Catena 15, 65–80 (1988).
Owens, P. N., Walling, D. E. & Leeks, G. J. L. Deposition and storage of fine-grained sediment within the main channel system of the River Tweed, Scotland. Earth Surf. Process. Landf. 24, 1061–1076 (1999).
Hurley, R., Rothwell, J. & Woodward, J. C. Metal contamination of bed sediments in the Irwell and upper Mersey catchments, northwest England: exploring the legacy of industry and urban growth. J. Soils Sediment. 17, 2648–2665 (2017).
Koelmans, A. A. et al. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 155, 410–422 (2019).
Hurley, R. R., Lusher, A. L., Olsen, M. & Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 52, 7409–7417 (2018).
Lusher, A. L., Bråte, I. L. N., Munno, K., Hurley, R. R. & Welden, N. A. Is it or isn’t it: the importance of visual classification in microplastic characterization. Appl. Spectrosc. 74, 1139–1153 (2020).
Primpke, S. et al. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal. Bioanal. Chem. 410, 5131–5141 (2018).
Gago, J., Galgani, F., Maes, T. & Thompson, R. Microplastics in seawater: recommendations from the Marine Strategy Framework Directive implementation process. Front. Mar. Sci. https://doi.org/10.3389/fmars.2016.00219 (2016).
Corcoran, P. L., Belontz, S. L., Ryan, K. & Walzak, M. J. Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environ. Sci. Technol. 54, 818–825 (2020).
Acknowledgements
We thank N. Scarle from the Cartographic Unit in the School of Environment, Education and Development at The University of Manchester, who drew all the diagrams. We also thank colleagues in the Department of Geography laboratories at The University of Manchester for technical support. K. Murnane assisted with the analysis of the effluent samples. We are grateful to the Environment Agency for providing river flow data for the River Tame.
Author information
Authors and Affiliations
Contributions
J.W. designed the study and wrote the paper. J.W., J.R. and J.L. carried out the field sampling. J.L. carried out the laboratory work and quantified the microplastics. R.H. carried out the FTIR analysis. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks Caroline Gauchotte-Lindsay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Microplastic contamination and microplastic assemblages at 40 sites on river channel beds around Manchester.
Note the catchment-wide decreases in microplastic storage on channel beds following the winter flooding of 2015/16. These catchments drain an area of about 1500 km2. Samples were collected from the 10 rivers in spring and early summer under low flow conditions2. Microplastic concentrations for the Denton hotspot on the River Tame actually increased between sampling periods from 48,300 to 72,400 microplastic particles per kg of fine bed sediment (FBS). While the pre-flooding assemblage at Denton was dominated by microbeads and fragments in roughly equal quantities, the acutely contaminated post-flooding bed assemblage was 98% microbeads. This shift in composition demonstrated that channel bed flushing did take place in this reach, but also that it was quickly followed by an influx of microbead-rich wastewater from a local point source.
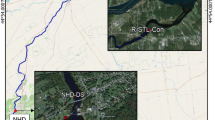
Extended Data Fig. 2 Industrial sites in the River Tame catchment that form potential sources of microplastics.
Note that this map is not exhaustive. The urban area is shown in grey. Industrial premises are concentrated in the river corridor between Stalybridge and Denton. Domestic wastewater is also an important source of microplastics. Wastewater from both industrial and domestic sources is processed in wastewater treatment plants but some industrial premises have consents to discharge directly to the urban drainage system or the river itself. Synthetic microbeads are used in a range of processes including blast cleaning and shot blasting. The 14 sample sites along the main river from 2019 are also shown along with the Denton Hotspot (DH) between Sites 6 and 7 that was sampled in 2015 and 2016. Base map redrawn from https://nrfa.ceh.ac.uk/data/station/info/69027.html.
Extended Data Fig. 3 Microplastic hotspots and the location of combined sewer overflows (CSOs) and wastewater treatments works (WwTW) along the main channel of the Tame.
CSOs are clustered in the main built up areas. CSO location data from United Utilities and our mapping. Hotspots are sites with a total microplastic concentration >15,000 particles per kg of fine bed sediment. The most heavily contaminated reaches (DH, 8, 9, 10, and 11) are located immediately downstream of wastewater treatment works or clusters of CSOs or both. The 2016 data are shown for the Denton hotspot (DH). In our 2015 survey of the wider region we identified 5 urban contamination hotspots in 10 rivers (Extended Data Fig. 1). Applying this threshold in the River Tame now produces 7 hotspots at sites 5, 6, 8, 9, 10, 11 along with the Denton reach from 2015/2016 (Fig. 2). It is very likely that more detailed sampling in the urban reaches (for example the cluster of CSOs between Sites 10 and 11) will identify further contamination hotspots. Base map redrawn from https://nrfa.ceh.ac.uk/data/station/info/69027.html.
Extended Data Fig. 4 An effluent release into low flows observed on the River Tame at Site 9.
a, This spill took place early (09:30) on Sunday 22 September 2019 with the river at low flow. b, The river water is heavily discoloured (milky silver grey) and the bed obscured even though water depth in the cylinder is <20 cm. The river water gave off a sweet pungent odour. This site is 150 metres downstream of the outlet from Ashton Wastewater Treatment Works (Fig. 2). The reaches immediately upstream of the WwTW were clear at this time. c, The channel bed of the Tame under normal low flow conditions. Site 9 yielded very high concentrations of microplastics on the channel bed and the highest concentrations of fibres in the bulk water sample (Fig. 3 and Supplementary Table 2). The composition of water sample 9 suggests that some fibres are retained in suspension even at low flow, but the bulk of the microplastic load is deposited on the channel bed in the reaches immediately downstream of their point of entry.
Supplementary information
Supplementary Information
Supplementary Tables 1–9.
Source data
Source Data Fig. 1
Source data
Source Data Fig. 2
Source data
Source Data Fig. 3
Source data
Source Data Extended Data Fig. 3
Source data
Rights and permissions
About this article
Cite this article
Woodward, J., Li, J., Rothwell, J. et al. Acute riverine microplastic contamination due to avoidable releases of untreated wastewater. Nat Sustain 4, 793–802 (2021). https://doi.org/10.1038/s41893-021-00718-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00718-2
This article is cited by
-
Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution
Nature Nanotechnology (2024)
-
A bio-based nanofibre hydrogel filter for sustainable water purification
Nature Sustainability (2024)
-
Prevalence of microplastics and fate in wastewater treatment plants: a review
Environmental Chemistry Letters (2024)
-
Polydimethylsiloxane-coated textiles with minimized microplastic pollution
Nature Sustainability (2023)
-
Long-distance atmospheric transport of microplastic fibres influenced by their shapes
Nature Geoscience (2023)