Abstract
We performed this cohort study to investigate whether the myocardial bridge (MB) affects the fat attenuation index (FAI) and to determine the optimal cardiac phase to measure the volume and the FAI of pericoronary adipose tissue (PCAT). The data of 300 patients who were diagnosed with MB of the left anterior descending (LAD) coronary artery were retrospectively analyzed. All of patients were divided into the MB group and the MB with atherosclerosis group. In addition, 104 patients with negative CCTA results were enrolled as the control group. There was no significant difference between FAI values measured in systole and diastole (P > 0.05). There was no significant difference in FAI among the MB group, the MB with atherosclerosis group, and the control group (P > 0.05). In MB with atherosclerosis group, LAD stenosis degree (< 50%) (OR = 0.186, 95% CI 0.036–0.960; P = 0.045) and MB located in the distal part of LAD opening (OR = 0.880, 95% CI 0.789–0.980; P = 0.020) were protective factors of FAI value. A distance (from the LAD opening to the proximal point of the MB) of 29.85 mm had the highest predictive value for abnormal FAI [area under the curve (AUC), 0.798], with a sensitivity of 81.1% and a specificity of 74.6%.
Similar content being viewed by others
Introduction
Coronary artery myocardial bridge (MB) is a congenital coronary artery abnormality1. The coronary artery and its branches are usually located in the epicardial adipose tissue. When one or part of the coronary artery tunnels through the myocardium, it is called “myocardial bridge”2. MB can occur in any coronary artery branch, but the common site is the proximal and middle segments of the left anterior descending branch (67–98%)3, which is more likely to be symptomatic.
Myocardial bridges affect the morphology and function of coronary arteries4, and change microcirculation in pericoronary fat, leading to inflammation5 and promoting the formation of atherosclerotic plaque in proximal segments of coronary arteries, even myocardial infarction6.
The fat attenuation index (FAI) of pericoronary adipose tissue (PCAT) is defined as the mean CT value of PCAT within the radial distance from the outer wall of the coronary artery equal to the diameter of the coronary artery. FAI is a novel imaging biomarker of coronary artery inflammation7 and can be measured on cardiac CT images.
At present, there is a lack of research on the relationship between myocardial bridge and FAI, and there is no research on the measurement of FAI value in cardiac systole or diastole. We employed an artificial intelligence software for quantitative assessment of the FAI. The computational principle of the software is based on CCTA images, using segmentation algorithms. According to the literature of The Lancet8, the software extends 4 mm outward from the vessel wall and uses a threshold of − 190 HU to − 30 HU for the pericoronary fat segmentation. Through the 3D rendering module, the quantitative information of the pericoronary fat is color-coded and mapped onto the MPR original image, CPR, probe images, and CPR straightening graph. This study aimed to quantitatively evaluate the FAI in patients with myocardial bridge and the relationship between FAI and the anatomical characteristics of MB, and to explore the factors influencing FAI measurement.
Method
Subjects
A total of 300 patients with only myocardial bridge of LAD were retrospectively enrolled, who received coronary computed tomography angiography (CCTA) examination in our hospital from January 2019 to June 2022. The flow chart is shown in Fig. 1.
Inclusion criteria:
-
1.
CCTA confirmed MB in left anterior descending artery;
-
2.
CCTA confirmed left anterior descending coronary atherosclerosis;
-
3.
CCTA confirmed that there was no myocardial bridge and coronary atherosclerosis in other coronary artery branches except the left anterior descending artery;
-
4.
There were no artifacts in systolic and diastolic images.
Exclusion criteria:
-
1.
Cardiomyopathy and valvular disease;
-
2.
Abnormal origin and termination of coronary artery, coronary aneurysm;
-
3.
The CCTA image quality was poor;
-
4.
Patients after coronary intervention and coronary artery bypass grafting;
-
5.
Cardiac pacemaker, occluder, artificial valve and other cardiac implants;
-
6.
CCTA confirmed myocardial bridge of other branches of coronary artery;
-
7.
CCTA confirmed coronary arteriosclerosis of other branches of coronary artery.
In addition, 104 patients who underwent a CCTA examination for chest tightness or a routine physical examination with negative CCTA results were enrolled as the control group. The flow chart is shown in Fig. 1.
Inclusion criteria:
-
1.
CTA result was negative;
-
2.
CCTA confirmed that there was no myocardial bridge in any coronary artery;
-
3.
There was no artifacts in systolic and diastolic images.
Exclusion criteria:
-
1.
CCTA confirmed that the origin and termination of coronary artery were abnormal;
-
2.
The CCTA image quality was poor.
Using a threshold of − 70.18, patients in both the MB group and the MB with atherosclerosis group were categorized into two groups: the normal FAI group (FAI < − 70.1) and the abnormal FAI group (FAI ≥ − 70.1). Subsequently, an analysis of the factors influencing FAI was conducted.
According to whether contained calcification, plaques were identified as three types: calcified, non-calcified, and mixed plaques.
The degree of coronary stenosis was classified as ≥ 50% and < 50%.
Ethics statement
This study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University. The informed consent was waived for this study by the Ethics Committee of the Second Hospital of Hebei Medical University. All methods were performed in accordance with the relevant guidelines and regulations.
Cardiac CT acquisition
A Phillips 256-slice spiral CT was used. The patient was in a supine position during the scan. A single end-expiratory breath-holding scan with retrospective ECG gating technique was performed. The breath-holding time was 5–7 s, and the scanning area began from 0.5 cm below the bifurcation of the autonomous trachea to the diaphragm of the heart. The intelligent tracking method was used to set the threshold of aortic layer to 150 HU, and the formal scan was started at 6 s after reaching the threshold. The non-ionic contrast agent Iohexol (350 mgI/ml) was injected with a double-barrel high-pressure injector at a rate of 4–5 ml/s. The dose was 0.8 ml/kg. Scanning parameters were as follows: tube current 320 mAs/revolution, tube voltage 120 kV, detector collimation 128 × 0.625, pitch 0.18, matrix 512 × 512, rotation time 330 ms, scanning field of vision 250 mm. The images of systolic phase (45% cardiac phase) and the images of diastolic phase (75% cardiac phase) were reconstructed.
Measurements
Measurement methods
Imported all the original images of CCTA into the post-processing workstation Phillips EBW 6.0, and used cross section, volume rendering (VR), multiplanar reconstruction (MPR) and surface reconstruction (CPR) to analyze the systolic and diastolic images. The anatomic parameters of myocardial bridge were measured by two radiologists with more than 5 years of CCTA related work experience, and each parameter was measured three times to get the average value.
The length of the myocardial bridge and the position of the myocardial bridge (the distance from the opening of the anterior descending coronary artery to the proximal point of the myocardial bridge) were measured on the diastolic phase images. The depth of the myocardial bridge (the thickness of the myocardium covering the coronary artery during diastole) was measured on the cross-sectional images, as shown in Fig. 2. When the maximum myocardial thickness is ≤ 1 mm, it is uniformly recorded as 1 mm9.
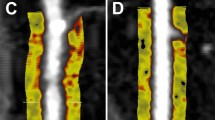
The method to quantify FAI and MB on cardiac computed tomography angiography images. (A) The axial plane image indicates the LAD and the FAI. (B) The axial plane image indicates the LAD and the MB. (C) Multiple axial plane images demonstrate the measurement of the FAI. (D) The FAI in the 40-mm proximal segment of the LAD is measured in a straightened view. (E) The axial plane image indicates the LAD and the MB depth. FAI fat attenuation index, LAD left anterior descending artery, MB myocardial bridging.
The reference formula is as follows10,11,12:
FAI and PCAT volume measurements
Based on an artificial intelligence software (Shukun, FAI, V1.7, Beijing, China), the 40-mm proximal segment of the LAD was traced and used to calculate FAI and PCAT volume. The software is based on various deep learning techniques, including CNN, and employs multiple deep learning composite networks to achieve fully automated vascular segmentation for a rapid and comprehensive diagnosis of all vessels. The cross-section images perpendicular to the vessel centerline were reconstructed, and the attenuation value of corresponding voxels around the artery is estimated as FAI calculation. The CT threshold of fat was − 190 to − 30 HU. PCAT was defined as adipose tissue within the distance from the outer wall of the vessel equal to the diameter of the vessel12,13. FAI was defined as the average CT attenuation value of PCAT. The FAI ≥ − 70.1 was abnormal13. A representative image of FAI analysis is shown in Fig. 2.
Statistical analysis
SPSS software (version 25.0) was used for statistical analysis of the data. The measurement data conforming to the normal distribution was expressed by mean ± standard deviation. The comparison between the two groups was conducted by t test, and the comparison between multiple groups was conducted by one-way analysis of variance. The measurement data that did not conform to the normal distribution were expressed as median (IQR). The comparison between the two groups used Mann–Whitney U test, and the comparison between multiple groups used Kruskal–Wallis H test. If there was statistical difference, the comparison between the two groups would be carried out afterwards. The categorical variables were expressed as frequency or percentage, and the differences between groups were compared by chi-square test. Binary logistic regression analysis was used to screen the influencing factors of abnormal FAI. A P value < 0.05 was considered statistically significant. The ROC curve of myocardial bridge location was plotted, and the area under the curve (AUC) was calculated, and the best cutoff value was obtained by Youden index. Jorden index = sensitivity + specificity − 1, the maximum value of Jorden index, the corresponding index is the best critical point.
Results
Participants characteristics
A total of 155 patients were enrolled in the MB group (median age 53 (13) years, 82 men, 73 women), and 145 patients were in the MB with atherosclerosis group (median age 55 (10) years, 83 men, 62 women). There were 104 patients in the control group (median age 54 (14) years, 25 men, 79 women). Baseline characteristics of the MB group, the MB with atherosclerosis group, and the control group were compared. The results showed that the incidence rate and smoking rate of men in the MB group and MB with atherosclerosis group are higher than those in the control group (P < 0.05) (Table 1).
For comparison of anatomical characteristics of MB among the groups, the results showed that the myocardial bridge depth in the MB group was greater than that in the MB with atherosclerosis group (P < 0.05), and there was no statistical difference in other indicators (P > 0.05) (Table 1).
In-group comparison of FAI and PCAT volumes
The results showed that there was no statistical difference in FAI in systole and in diastole (P > 0.05) (Table 1; Fig. 3A). The volumes of PCAT in diastole was greater than that in systole (P < 0.05) (Table 1; Fig. 3B).
Comparison of the FAI and PCAT values in diastole and in systole in-group. (A) FAI was compared in-group. It showed no statistic difference. (B) PCAT volume was compared in-group. It was significantly different in diastole and in systole. FAI fat attenuation index, PCAT pericoronary adipose tissue, Normal the control group without MB, MB myocardial bridging, MBLA myocardial bridging combined with LAD atherosclerosis, LAD left anterior descending coronary artery.
Comparison of FAI and the PCAT volumes among the MB group, the MB with atherosclerosis group, and the control group
The results showed that there was no significant difference in FAI among the three groups in systole and in diastole (P > 0.05) (Table 1; Fig. 4A).
Comparison of the FAI and PCAT values in the diastolic and systolic phase. (A) Comparison of the FAI values in the diastolic and systolic phase. (B) Comparison of the PCAT volumes in the diastolic and systolic phase. Normal the control group without MB, MB myocardial bridging, MBLA myocardial bridging combined with LAD atherosclerosis, FAI fat attenuation index, PCAT pericoronary adipose tissue, LAD left anterior descending coronary artery; *P < 0.05; **P < 0.01.
The results showed that in the diastole, the PCAT volume of normal group was higher than that of myocardial bridge group and myocardial bridge with atherosclerosis group (P < 0.05), but there was no statistical difference between myocardial bridge group and myocardial bridge with atherosclerosis group. In the systolic period, the PCAT volume in the normal group was higher than that in the myocardial bridge group (P < 0.05), but there was no difference between the normal group and the myocardial bridge with atherosclerosis group (Table 1; Fig. 4B).
Analysis of factors influencing FAI in the MB group
Patients in the MB group were divided into the normal FAI group and the abnormal FAI group. There was no statistically significant difference in clinical characteristics and myocardial bridge anatomic characteristics between the two groups (P > 0.05; Table 2).
Analysis of factors influencing FAI in the MB with atherosclerosis group
Patients in the MB with atherosclerosis group were divided into the normal FAI group and the abnormal FAI group. There were statistically significant differences in BMI, length, depth, location and MBMI of myocardial bridge between the two groups (Table 3). The above variables and variables of clinical significance or reported significance were included in the multivariate binary logistic regression analysis. The results showed that LAD stenosis degree < 50% (OR = 0.135, 95% CI 0.027–0.668; P = 0.014) and MB position (OR = 0.880, 95% CI 0.789–0.980; P = 0.020) were protective factors for abnormal FAI (Fig. 5; Table 4).
Predictive value of MB location
The ROC curve analysis showed that a distance (from the LAD opening to the proximal point of the MB) of 29.85 mm had the highest predictive value for abnormal FAI [area under the curve (AUC), 0.798], with a sensitivity of 81.1% and a specificity of 74.6% (Fig. 6). MB closer to the LAD opening will incur a relatively higher risk.
Discussion
PCAT is not only the structural support tissue of blood vessels, but also the endocrine organ with active metabolism, which can produce various pro-inflammatory and anti-inflammatory factors13. PCAT is considered to be the key factor to maintain the steady state of cardiovascular physiological function and lead to cardiovascular disease, and can respond to coronary artery inflammation and change the shape and secretion characteristics through the “from inside to outside” signal pathway. These changes can be identified by CTA non-invasive imaging14. By calculating FAI, we can non-invasive measure coronary artery inflammation, capture changes in fat attenuation around blood vessels, and predict cardiovascular risk of patients14.
Myocardial bridge was first proposed by Dr. Henrick Reyman in 173215. At that time, it was considered as a silent anatomical variation. Most people had no obvious symptoms, but in patients with symptoms, it could lead to serious cardiovascular events16, such as serious adverse coronary artery events, myocardial infarction, myocardial ischemia and sudden cardiac death, and the incidence was gradually increasing17,18,19.
This study found that male patients with myocardial bridge had a higher incidence rate and smoking rate, which was consistent with Aydar and Podolec’s research20,21. The higher incidence of MB among smokers may be related to the increasing risk of coronary artery spasm by smoking21.
Although there have been studies on PCAT volume and FAI value, there is no comparison between systolic and diastolic measurements of those parameters. Our study found that there was no significant difference in FAI values between systolic and diastolic phases measurements, but there was difference in PCAT volumes. The reason for this may be that FAI is the average CT attenuation of PCAT after correction and weighting22, which quantifies coronary artery inflammation through the difference of PCAT attenuation gradient8, and adjusts some factors affecting PCAT. Therefore, the measurement of FAI value is not affected by the cardiac cycle phase, and may be more suitable than PCAT volume for the evaluation of pericoronary fatty lesions. Oikonomou et al.8 showed that FAI was more accurate in predicting cardiovascular diseases and had higher prediction ability. In the diastole, the PCAT volume of normal group was higher than that of myocardial bridge group and myocardial bridge with atherosclerosis group. We speculate that measuring PCAT volume during diastole may be more conducive to detecting changes in pericoronary adipose tissue (PCAT) associated with myocardial bridge or atherosclerotic plaques.
This study found that the clinical symptoms, risk factors and myocardial bridge anatomy were not the influencing factors of FAI abnormalities in patients with myocardial bridge. It shows that myocardial bridge has no effect on the FAI value of pericoronal fat. However, the abnormality of FAI in patients with both myocardial bridge and coronary atherosclerosis is related to the location of myocardial bridge and the degree of vascular stenosis. The closer the location of myocardial bridge to the opening of the LAD, and the greater the degree of stenosis, the more likely it will lead to abnormal FAI values. In other words, pericoronary steatoinflammation increases at the proximal end of the myocardial bridge only in the presence of large atherosclerotic plaques. It is speculated that the closer the myocardial bridge is to the LAD orifice, the greater the intimal thickening at the proximal end23, which causes changes in the hemodynamics of the proximal vessel, thus leading to the formation of atherosclerotic plaque24, increased inflammation and changes in FAI values at the proximal end of the myocardial bridge.
Vasa vasorum (VV) in the proximal and distal segments of myocardial bridge increased4. The increase of VV formation is considered as an inflammatory change of adventitia4. The closer the myocardial bridge is to the port of the left coronary artery, the greater the intimal thickening at the proximal end23. Generally, coronary atherosclerosis does not occur at the myocardial bridge segment, and atherosclerotic plaque often occurs at the proximal end of the myocardial bridge24,25. Local hemodynamic disorder caused by muscle contraction of the myocardial bridge may play a role in plaque formation24.
Our research has certain limitations. First of all, this study was a single-center study with a limited number of cases. Second, this study only focused on myocardial bridges located in LAD. Although this is the most common location of myocardial bridges, our data may not be applicable to myocardial bridges in other arteries. Third, the FAI value measured in this study is only the average density and cannot fully reflect the structural characteristics of the fat around the myocardial bridge. In addition, artificial intelligence technology combined with radiomics is expected to provide accurate evaluation of FAI in the future26,27.
Conclusion
Myocardial bridge may affect the volume of pericoronal fat, but it does not affect the pericoronal fat attenuation index. When myocardial bridge patients with LAD atherosclerosis, the degree of coronary artery stenosis < 50% and the location of myocardial bridge away from the LAD opening are protective factors of FAI value.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- CCTA:
-
Coronary computed tomography angiography
- LAD:
-
Left anterior descending coronary artery
- MB:
-
Myocardial bridging
- FAI:
-
Fat attenuation index
- PCAT:
-
Epicardial adipose tissue
- OR:
-
Odds ratio
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
References
Roberts, W. et al. Myocardial bridges: A meta-analysis. Clin. Anat. 34, 685–709 (2021).
Santucci, A. et al. The myocardial bridge: Incidence, diagnosis, and prognosis of a pathology of uncertain clinical significance. Eur. Heart J. Suppl. 24, 161–167 (2022).
Sternheim, D. et al. Myocardial bridging: Diagnosis, functional assessment, and management: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78, 2196–2212 (2021).
Nishimiya, K. et al. Absence of adventitial vasa vasorum formation at the coronary segment with myocardial bridge—An optical coherence tomography study. Int. J. Cardiol. 250, 275–277 (2018).
McLaughlin, T. et al. Relationship between coronary atheroma, epicardial adipose tissue inflammation, and adipocyte differentiation across the human myocardial bridge. J. Am. Heart Assoc. 10, e021003 (2021).
Zhou, F. et al. Machine learning using CT-FFR predicts proximal atherosclerotic plaque formation associated with lad myocardial bridging. JACC Cardiovasc. Imaging 12, 1591–1593 (2019).
Goeller, M. et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 20, 636–643 (2019).
Oikonomou, E. K. et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the crisp CT study): A post-hoc analysis of prospective outcome data. Lancet (London, England) 392, 929–939 (2018).
Yu, M. et al. Assessment of myocardial bridge by cardiac CT: Intracoronary transluminal attenuation gradient derived from diastolic phase predicts systolic compression. Korean J. Radiol. 18, 655–663 (2017).
Liu, S. H. et al. Myocardial bridging on dual-source computed tomography: Degree of systolic compression of mural coronary artery correlating with length and depth of the myocardial bridge. Clin. Imaging 34, 83–88 (2010).
Konen, E. et al. The prevalence and anatomical patterns of intramuscular coronary arteries: A coronary computed tomography angiographic study. J. Am. Coll. Cardiol. 49, 587–593 (2007).
Ishikawa, Y. et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation 120, 376–383 (2009).
Sacks, H. S. & Fain, J. N. Human epicardial adipose tissue: A review. Am. Heart J. 153, 907–917 (2007).
Antonopoulos, A. S. et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 9, eaa12658 (2017).
Reyman, H. C. Dissertatio de vasis cordis propriis. Bibl. Anat. 2, 359–378 (1737).
Khan, M. O. et al. Colocalization of coronary plaque with wall shear stress in myocardial bridge patients. Cardiovasc. Eng. Technol. 13, 797–807 (2022).
Hostiuc, S., Rusu, M. C., Hostiuc, M., Negoi, R. I. & Negoi, I. Cardiovascular consequences of myocardial bridging: A meta-analysis and meta-regression. Sci. Rep. 7, 14644 (2017).
Abe, T., Jujo, K. & Matsukage, T. Myocardial bridging may shed light on one aspect of unexplained myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. Acute Cardiovasc. Care 11, 508–509 (2022).
Bhogal, S., Waksman, R. & Hashim, H. Going under the bridge: Unmasking ischaemia and endothelial dysfunction of myocardial bridging: A case report. Eur. Heart J. Case Rep. 7, ytad047 (2023).
Aydar, Y. et al. Gender differences in the types and frequency of coronary artery anomalies. Tohoku J. Exp. Med. 225, 239–247 (2011).
Podolec, J. et al. Prevalence and clinical presentation of myocardial bridge on the basis of the national polish percutaneous interventions registry and the classification of rare cardiovascular diseases. Kardiologia Polska 77, 465–470 (2018).
Antoniades, C., Antonopoulos, A. S. & Deanfield, J. Imaging residual inflammatory cardiovascular risk. Eur. Heart J. 41, 748–758 (2020).
Ishii, T., Ishikawa, Y. & Akasaka, Y. Myocardial bridge as a structure of “double-edged sword” for the coronary artery. Ann. Vasc. Dis. 7, 99–108 (2014).
Yamada, R. et al. Functional versus anatomic assessment of myocardial bridging by intravascular ultrasound: Impact of arterial compression on proximal atherosclerotic plaque. J. Am. Heart Assoc. 5, e001735 (2016).
Forsdahl, S. H. et al. Myocardial bridges on coronary computed tomography angiography—Correlation with intravascular ultrasound and fractional flow reserve. Circ. J. 81, 1894–1900 (2017).
Antoniades, C., Kotanidis, C. P. & Berman, D. S. State-of-the-art review article. Atherosclerosis affecting fat: What can we learn by imaging perivascular adipose tissue?. J. Cardiovasc. Comput. Tomogr. 13, 288–296 (2019).
Huo, Y., Svendsen, M., Choy, J. S., Zhang, Z. D. & Kassab, G. S. A validated predictive model of coronary fractional flow reserve. J. R. Soc. Interface 9, 1325–1338 (2012).
Author information
Authors and Affiliations
Contributions
Data collection and analysis: D.Z., X.T. Study design: C.-Y.L. Statistics: M.-Y.L., H.-W.Z. Supervision: C.-Y.L. Figure preparation: Y.Y., T.P. Approval of the paper: all authors. Writing of the draft: D.Z., X.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, D., Tian, X., Li, MY. et al. Quantitative analysis of the relationship between the myocardial bridge and the FAI of pericoronal fat on computed tomography. Sci Rep 14, 5976 (2024). https://doi.org/10.1038/s41598-024-55005-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55005-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.