Abstract
Understanding the bioavailability and phytotoxicity of Carbendazim (MBC) bound residues (BR) in soils incubated with different Superabsorbent polymer (SAP) amendment on succeeding crops is essential to assess their environmental fate and risks. In our research, we studied the morphological characteristics and 14C-accumulation of Chinese cabbage and released BR in three typical cultivated soils. The plant dry weight was in order of superabsorbent-hydrogels formulations (HMBC) > MBC > MBC and SAP (MBC-SAP) at 35 d in basic soil 3 (S3), with 675.40 ± 29.07 mg/plant.d.w, 575.93 ± 25.35 mg/plant.d.w and 427.86 ± 18.79 mg/plant.d.w. The whole plant accumulated 2-fold more BR when grew in neutral soil 2 (S2) treated with SAP than MBC at 7 d. The root accumulated a greater proportion of 14C-MBC residue than shoot, with order of MBC-SAP > MBC > HMBC at 21d. The results indicate MBC-BR could be released and accumulated in plant. HMBC promoted the Chinese cabbage growth with lowest 14C accumulation, while MBC-SAP inhibited plant growth with the highest 14C uptake. The released BR rate was 61.43 ± 3.75% of initial BR in MBC-SAP, with 2-fold higher than MBC and HMBC. It is assumed HMBC could be a potential environmentally friendly measure for rational use of pesticides in future.
Similar content being viewed by others
Introduction
Pesticides are ubiquitous chemicals in the environment, and are usually used to control crop disease and maintain the products. When pesticides are applied to the field and undergo degradation, their metabolites and they could bind to organic or mineral constituents of soil, and form the non-extractable residue (bound residue) in soil1. Bound residue (BR) is generally regarded as the soil detoxification process, which involves chemicals could permanently bind to soil matrix, and are no longer bioavailable and bio-accessible to organisms. However, some studies reported that BR could be released via microbial activity and physic-chemical mechanism, and accumulated in living tissues and food webs, posing high threat to human health2. Han et al. found 14C-labeled residue of ZJ0273 was released from the BR-amended soil upon planting, and rice seedling took up the 14C from soil contaminated with compound residues and was inhibited to grow3. Gao et al. detected a clear uptake, accumulation and translocation of phenanthrene and pyrene by ryegrass, and significant phytoavailabilty of bound-PAH residue4. Liu et al. also demonstrated that the earthworms accumulated 31.5% of the total radioactivity 14C-BR-RM5 after exposure to the BRs of 14C-CYC. Thus, the food safety issue of agricultural products originated from contaminated soils should be given a public concern. The potential release, bioaccumulation and phytotoxicity of the BR to succeeding non-target organism are always important topics for understanding the environment and human food safety impact of pesticides.
Soils are the vital resources that provide life-supporting services of food production, water cleaning and habits for human and wildlife. Intensified agricultural production has deteriorated the soil quality and led to the increasing amount of anthropogenic contaminants to the environment. The contamination of agricultural soils has a plethora of negative impacts on food production and agroecosysterm service. Soil amendments or water adsorbents are applied in the agriculture to realize the coupled effects of water, fertilizer and pesticides. Superabsorbent polymer (SAP) is common soil conditioner to hold the soil moisture and improve soil property in agriculture6,7,8. Moreover, SAP also has been employed in combination with pesticides as a new formulation to control their release rates and to promote the efficient use of both pesticides and water9,10,11. Therefore, SAP is usually coexisting with pesticides in agriculture in different forms of amendments or new pesticides formulations. However, relatively less work has been conducted to study the soil environmental fate of pesticides when amended with SAP. Yang et al. found that when SAP coexisted with pesticides in soil, the BR of MBC was increased when soil spiked with MBC and SAP (MBC-SAP), and decreased in term of superabsorbent hydrogels (SHs) formulations (HMBC)12,13. Nowadays, the potential for the release and subsequent availability and phytotoxic effects of BR when pesticides coexist with SAP or encapsulate as new formulations remain poorly understood, especially for the succeeding crops and food contamination in agriculture production. In fact, the bioaccumulation of pesticides amended with SAP or SHs-formulations in plants, especially crops, can cause potential risks in the food chain and human health. Therefore, it is important to clarify the bio-effect of pesticides-bound residue to crops and to provide safe agricultural products.
Chinese cabbage is a popular green leafy crop, and is widely vegetated for its rich nutritional and favorable taste. The shoot and leaf can be eaten at the seedling stage, and the seed can be contacted into soil at the ripening stage. Chinese cabbage seedlings are also common succeeding crops in fields. MBC, a broad spectrum benzimidazole fungicide, is usually used against fungi which affect crops, fruits and vegetables. However, MBC can be accumulated in plant and transferred to different parts, interfere mitosis of bacterial cell and inhibit its growth14,15. For example, Alicja Lewandowska et al. found that MBC residue can be taken up by plants from extractable residue in soils16. Thus, the primary objective of this study is to evaluate the plant availability and phytotoxicity of MBC-BR after the amendment with SAP to Chinese cabbage seedlings. We planted the Chinese cabbage seed into the MBC-BR amended soil with HMBC, MBC-SAP and MBC treatment to reveal the effect of BR on the growth of Chinese cabbage by measuring the plant height, plant weight, tracing the 14C content distribution and accumulation patterns in the different part of Chinese cabbage for safety assessment. Furthermore, we detected the BR release rate, extractable residue and bound residue in soil after the Chinese cabbage cultivation to demonstrate the soil safety.
Results
The effects of BR on cabbage growth
The growth parameters of cabbage, including plant height, dry weight of shoot, root, flower, and total plant were determined to evaluate the soil BR effects on plant growth (Table 1). Cabbage gradually grew with the incubation time in all treatments. Significant difference was observed in the growth of cabbage between three tested soils, indicating cabbage growth may be closely related with soil property and microbes. Cabbage didn’t have a favorable growth in S1 and grew till 21 d. The dry weight of total plant was all below 5.00 mg for each plant, and the height of whole plant was around 1.50 cm in all treatments, which was significantly lower than those in S2 and S3 (p < 0.05). Meanwhile, there was no significant difference between HMBC, MBC-SAP, MBC treatment and blank control in S1 (p > 0.05).
On the contrary, cabbage underwent an appreciate growth in S2 and S3 and blossomed at 35 d. There was a time-dependent biomass increase trend during the whole incubation. Compared with blank control, the growth of cabbage was promoted in all treatments spiked with MBC-BR in S2. Moreover, the dry weights (shoot, root, flower and total) of plant in both HMBC and MBC-SAP treatments were higher than those in MBC treatment (p < 0.05). Till 35 d, the total dry weight of cabbage was 930.10 ± 22.89, 704.43 ± 16.17 mg/plant in HMBC and MBC-SAP treatments, separately. Meanwhile, the dry weight of the edible leafy portion (shoot part) of cabbage was significantly higher in HMBC treatment than those in MBC-SAP and MBC control (p < 0.05), with 571.97 ± 21.40 mg/plant contrast to 398.27 ± 17.59 mg/plant and 365.23 ± 11.31 mg/plant, respectively. We also found the similar result in S3. The biomass of cabbage in HMBC treatment was significantly higher than those in the MBC-SAP and MBC treatments, with the highest height for 25.14 ± 0.35 cm, dry weight of shoot for 443.43 ± 23.50 mg/plant and the total dry weight for 675.40 ± 29.07 mg/plant at day 35. However, for the MBC-SAP treatment in S3, the biomass of cabbage was lower than the MBC control but statistically equal to those of the blank control without MBC-BR, with the lowest height for 18.32 ± 0.59 cm, dry weight of root for 110.73 ± 7.07 mg/plant and the total dry weight for 427.86 ± 18.79 mg/plant at day 35.
The distribution of 14C radioactivity in cabbage in soil amended with BR of MBC
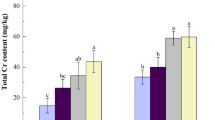
During the cabbage growth in BR spiked soil, the 14C radioactivity in vegetable tissues were detected, which means cabbage could absorb the 14C-compound from soil spiked with released BR. Analysis of 14C radioactivity in cabbage showed the bioaccumulation of 14C content in shoot, root and the whole plant were mostly higher in the initial incubation than those in the final phase planting (Fig. 1). Figure 1a shows the 14C distribution in cabbage in S1. Obviously, a majority of 14C content was accumulated in root, and less 14C existed in shoot, suggesting the root was the main enrichment site of soil released BR. Compared with MBC control (36.50 ± 8.14 μg/g), the 14C content of whole plant was significantly lower in MBC-SAP and HMBC treatments at 7 d, with the corresponding values 19.02 ± 3.46 μg/g and 18.07 ± 0.55 μg/g on the dry biomass basis, separately. For the shoot, 14C level in HMBC treatment was significantly lower than those in the MBC-SAP and MBC treatment (p < 0.05). While, for the 14C accumulation in root, there was no difference between the HMBC treatment and MBC control (p > 0.05), those much higher than that in MBC-SAP treatment. However, when the cabbage planted until 21 d, no significant difference was observed between three different treatments for the 14C distribution in plant.
However, cabbage could grow vigorously and blossomed in 35 d in S2. We detected the 14C accumulation in the flowers part (Fig. 1b). The 14C content of flowers was 2.14 ± 0.33 μg/g, 2.10 ± 0.23 μg/g and 1.53 ± 0.23 μg/g in MBC, MBC-SAP and HMBC treatments, respectively. There was a decrease of 14C-bioaccumulation in whole plant, shoot and root of cabbage with the extension of incubation. When cabbage was cultured for 35 days, the 14C-distribution in whole plant was decreased from the initials 4.61 ± 0.53 ug/g to 1.04 ± 0.08 ug/g for MBC treatment, 4.49 ± 1.23 μg/g to 1.30 ± 0.07 μg/g for MBC-SAP treatment, 1.05 ± 0.46 to 0.58 ± 0.03 μg/g for HMBC treatment, separately. Obviously, the 14C accumulation in whole plant was the lowest in HMBC treatment. However, in MBC-SAP treatment, the cabbage could absorb much more released BR from soil in whole tissue during the incubation, with 16.41 ± 4.33 μg/g, and 4.19 ± 0.19 μg/g at 7d and 21d, separately, compared with MBC control (7.35 ± 0.58 μg/g and 2.90 ± 0.12 μg/g). For the root accumulation, there was no significant difference between the HMBC and MBC treatments during the 21 days incubation (p > 0.05). While the 14C content in MBC-SAP treatments decreased firstly then increased at 21 d. At 100d, the 14C accumulation in root was followed in order of MBC > MBC-SAP > HMBC treatment.
Similarly, we found the same trend of 14C bioaccumulation in cabbage in S3. An increase in 14C content in whole plant was consistent with the decrease in plant height and dry weight. At 7 d, the 14C content of whole plant in HMBC treatment was higher than in the MBC-SAP and MBC treatments corresponding to the lower biomass of total plant (Table 1 & Fig. 1c). This suggested the plant growth inhibition was due to the accumulation of the released chemicals from BR of MBC in soils. When cabbage cultured at 21 d and 35 d, the whole plant 14C in MBC-SAP was higher than those in MBC and HMBC treatments. For instance, when cultured at 35d, the 14C level in whole plant was 2.19 ± 0.11 μg/g in MBC-SAP treatment, significantly higher than the MBC (0.92 ± 0.07 μg/g) and HMBC treatment (0.75 ± 0.02 μg/g), respectively (p < 0.05). HMBC treatment has the lowest 14C accumulation in the whole plant among all the treatments. We also have the similar change trend for 14C bioaccumulation in shoot, with an order in HMBC < MBC < MBC-SAP treatment. For the root, the 14C activity reached a maximum at 21 d in the MBC-SAP treatment (21.21 ± 2.70 μg/g), which was significantly higher than the MBC (14.59 ± 1.14 μg/g) and HMBC treatments (15.70 ± 1.15 μg/g) (p < 0.05).
Determination of released BR in different SAP amendment treatments
After the cabbage cultivation and the subsequent extraction of soil, the decreased radioactivity in whole soil was the fraction of the released BR. The fraction of BR release after cabbage planting was 51.87 ± 0.28%, 47.91 ± 3.34%, and 51.57 ± 1.07% of the initial applied activity in S1, respectively for MBC, MBC-SAP and HMBC treatment at 7 d (Fig. 2a). There was no significant change of the released BR during the incubation in each BR-amended treatment (p > 0.05).
In additional, the soils amended with MBC-BR after cabbage culture was extracted, and the extractable part was combined and nominated as extractable residue (ER). ER decreased with incubation time, and there was significant difference between these three treatments (p < 0.05), with following the order of HMBC > MBC > MBC-SAP (Fig. 3a) in S1. Compared to MBC control, more ER was detected in HMBC treatment, corresponding to the lower 14C bioaccumulation in plant mostly concentrated on the root. The lowest amount of ER was found in MBC-SAP treatment. Furthermore, there were still high amount of BR in soil after extraction and cabbage culture at the end of incubation, with 51.32 ± 3.29%, 60.03 ± 6.69% and 53.12 ± 2.04% respectively, for MBC, MBC-SAP and HMBC treatment (Fig. 3a). There was no significant difference between three treatments (p > 0.05).
In S2, a little ER was extracted from soil after cabbage planting during the incubation, with approximately 0.13~3.17% of the initial BR amount (Fig. 3b). This suggested mostly released BR was taken up by plant or turned into BR or released as CO2 into atmosphere. During incubation, the lowest amount of BR was found after extraction in the MBC-SAP treatment (38.57 ± 3.75%) when compared with MBC and HMBC treatment (61.76 ± 5.26% and 53.44 ± 3.46%, respectively). But much larger amount of the released BR was calculated in MBC-SAP treatment, with the relative enhancement of 37.74% and 24.21%, compared to the MBC treatment and HMBC treatment, respectively at 100 d (Fig. 2b). Similarly, we detected the same result in S3 in terms of released BR rate. The released rate of BR was 51.66 ± 2.70%, 60.70 ± 1.40%, and 51.57 ± 1.29%, respectively in MBC, MBC-SAP and HMBC treatment at 35 d (Fig. 2c). Larger amount of released BR in MBC-SAP treatment may result in the higher amount of 14C per dry biomass of cabbage, and the inhibition of cabbage growth, corresponding to our above results. However, there was no significant difference between MBC and HMBC treatment in terms of released BR rate (p > 0.05). In additional, at the end of incubation, the amount of soil BR after extraction and cabbage growing was 48.34 ± 2.70%, 39.30 ± 1.39% and 48.43 ± 1.29% in MBC, MBC-SAP and HMBC treatment, respectively. Obviously, the lowest amount of BR existed in MBC-SAP treatment.
Discussion
Superabsorbent hydrogel (SAP) usually are used as soil conditioners and chemical formulations in agriculture to maintain soil moisture and enhance the pesticide efficiency17. However, some research showed when pesticides coexisted with SAP, the environmental fate and transformation of pesticides could be changed, especially for bound residue12,13,18. The bound residue could be released during further agricultural practices and may influence the succeeding crop and soil management2,19.
In this study, we studied the plant availability and phytotoxicity of MBC-BR in three typical cultivated soils amended with SAP and SHs-formulations. Different SAP amendments could affect the cabbage growth differently, including plant height, dry weight of shoot, root, flower, and total plant. For acidic clayey soil S1, cabbage grew till 21 d, with lowest plant height and dry weight of whole plant compared with others soils, with no significant difference between MBC, MBC-SAP and HMBC treatment during the incubation (p > 0.05). This is might be due to S1 with barren microbes and low pH value and electrical conductivity, which is not favorable for the planting crops, and cabbage could not grow healthily. While for neutral loamy soil S2 and basic saline soil S3 with rich microbes, organic matter and high electrical conductivity, cabbage underwent the favorable growth, and there was the highest plant height and dry weight of whole plant in HMBC treatment when compared with MBC treatment. MBC-BR in SHs-encapsulated treatment and SAP amendment treatment could enhance the growth of cabbage in S2, especially for SHs-encapsulated treatment. SAP, as soil conditioner, could retain water and keep soil moisture, and hold the water for the plant growing during incubation. In addition, SAP could be a favorable nutrition sources for soil microorganism. It might enhance the activity and biodiversity of microbes to some extent20,21. But for MBC-SAP treatment, the growth of cabbage was inhibited during the incubation, especially for S3. However, different forms of SAP amendments could affect cabbage growth in BR amended soil differently. The different bio-effect on cabbage in soil might also related with the different BR release rates. Han et al. demonstrated the plant height and dry weight decreased as BR amendment increased, and the herbicide ZJ0273 and its metabolites from released BR imposed serious phytotoxic effects on rice plant3.
The dry weights of cabbage shoot and whole plant in HMBC treatment were increased substantially compared with MBC-SAP and MBC alone treatment in all soils. Based on previous results, SHs-encapsulated formulation could significantly increase the dissipation and mineralization of MBC, and reduce the BR substantially13. With the environment friendly transformation fate of HMBC and favorable biomass for cabbage, SHs-encapsulated formulation might be a good way to spike the pesticide into the environment and cut down the potential hazards to agro-ecosysterm.
Meanwhile, the 14C-distribution in cabbage was detected in three treatments, there was an increase of the 14C-bioaccumulation in cabbage whole plant, shoot and root with the incubation in all treatments. The 14C bioaccumulation of cabbage is closely related with the biomass of cabbage and the amount of released BR during the incubation. 14C content in whole plant and shoot part of cabbage were the lowest in HMBC treatment compared with MBC and MBC-SAP treatments, suggesting HMBC could reduce the released BR accumulation in cabbage, especially for the edible shoot. However, cabbage could absorb much more released BR from soil in whole tissue and edible part of cabbage during the incubation in MBC-SAP treatment. According to the European Food Safety Authority, the maximum residue level (MRLs) of MBC in sugar beets and vegetable is <5 mg/kg in vegetable. For cabbage cultured at initial stage in neutral soil S2 and basic soil S3, the 14C-bioaccumulation in plant and edible part has been exceeded the standard value, and are higher in MBC-SAP than MBC and HMBC treatment. The acceptable daily intake (ADI) index is 0.02 mg/kg bw/d (body weight per day). So for an adult with 100 kg, the maximum ingestion of MBC per day is 2 mg. Thus it is important for us, and we should give priority attention to the food safety especially for crop grown in the soil spiked with SAP. It seems HMBC may be the potential way to spike pesticides into environment without the high pesticide residue bioaccumulation in cabbage and with safe fate in ecological environment12,13.
Based on BR release results, we detected higher released BR in MBC-SAP treatment. This plant growth inhibition might be due to the accumulation of the released chemicals from MBC-BR in soils. Different SAP amended treatments could trigger the remarkable different effects on soil BR release after sowing the succeeding crops. Based on our previous results of the fate of carbendazim amended with SAP and SHs-formulation, there was still higher amount of initial carbendazim BR in MBC-SAP treatment compared with MBC control and HMBC12,13. It seems that when we spike the carbendazim and SAP amendment into soil, there will be much more BR in soil and this residue is more readily released when we planted cabbage in the BR-amended soil. Compared with HMBC treatment, the MBC-SAP treatment did not have safer environmental effect in soil and crops. Gevao et al. indicated BR can be released by physicochemicals mechanism or through biochemical process22. Agriculture practices and the introduction of certain chemicals that may change soil texture and property could result in BR releasing. SAP could change the soil texture and keep moisture, and also alter the soil microorganism abundance and biodiversity23. Physical entrapment of SAP, MBC, and metabolites in soil organic and inorganic matrices stimulated by microorganisms could lead to the formation of organoclay complexes and soil aggregates, with the soil BR increasing during the incubation. Meanwhile, SAP also could interact with the surface active of MBC and its metabolites due to some polar groups of –OH, –COOH, and –NH222. These physical and chemical changes could alter the BR substance and may be released upon planting crops. Gao et al. suggested the plant root exudates usually play an important role in the environmental processing of organic pollutants, which could release the BR in soils4.
After the cabbage incubation, the soil bound residue differed in different SAP amendments and soils, following the order of MBC-SAP < HMBC = MBC treatment at the end of incubation in the neutral soil S2 and basic soil S3. Though BR was low in soil in MBC-SAP treatment, we should pay more attention on the further BR release in terms of succeeding crops and soil management. Above all, compared to the MBC treatment, cabbage could accumulate the released BR from soil more easily in MBC-SAP treatment, and less in HMBC treatment. This behavior might be closely related with the amount of BR which was released from the soil during the plant culture.
Conclusion
Our findings show that high attention should be given to the environmental risk assessment of pesticides BR when pesticides spiked with SAP amendments. When SAP is amended into the soil environment to keep soil moisture and enhance pesticides efficiency, in comparison, the SHs-formulation seems safer than SAP amendment for the utility of pesticides in the environment. SHs-encapsulated formulations may be a promising efficient and environmentally friendly method to reduce pesticide residue and keep the natural ecosystem and human health. In addition, the toxicity effects and long-term stability of other pesticides-SHs encapsulated formulations also need to be carried out to achieve the safely exploitation in further research.
Materials and Methods
Chemicals and reagents
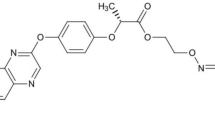
14C-MBC (methyl-2-benzimidazole carbamate), with 14C-labeled imidazole ring (Fig. 4) was obtained from ChemDepo Incorp. (Camarillo, CA). The radiochemical and chemical purity of 14C-MBC was >97% and specific activity was 1.89 × 109 Bq mmol−1. Non-labeled MBC (chemical purity >96%) was purchased from Sigma-Aldrich (Munich, Germany). The 14C-MBC stock solution was prepared by mixing the labeled Carbendazim and non-labeled in methanol at a final specific activity of 4.625 × 104 Bq mg−1. Acetonitrile and glacial acetic acid were HPLC grade agents. All solvents such as hydrochloric acid, ethanolamine, sodium hydroxide, methanol, and glycol ether were of analytical grade. The cocktail A solution contained 0.5 g of 1,4-bis (5-phenyloxazoly-2-yl)-benzene (POPOP), 7.0 g of 2,5-diphenyloxazole (PPO), 650 mL of dimethyl benzene and 350 mL of glycol ether. Cocktail B contained 0.5 g of POPOP, 7.0 g of PPO, 550 mL of dimethyl benzene, 275 mL of glycol ether, and 175 mL of ethanolamine.
Soil, SAP and H-14C-MBC
Soils were sampled from the first horizon (0–15 cm) in agricultural fields in Hangzhou (fluvio-marine yellow loamy soil), Cixi (coastal saline soil), and Longyou (red clay soil), Zhejiang Province, China, which are abbreviated separately herein as S1, S2, and S3. All soils were air-dried, sieved through a 2 mm mesh and stored at room temperature before use. The main physico-chemical properties of soils were determined using standard methods and summarized in Table 2.
SAP, starch-graft-polyacrylamide (St-g-PAM) superabsorbent cross-linked by N,N-methyl bisacrylamide were synthsized via 10 MeV simultaneous electron beam irradiation at room temperature and subsequent alkaline hydrolysis. The swelling ratio of SAP (deionized water) was approximately 1000 g g−1. SAP was dried at room temperature in a vacuum drying apparatus24.
The H-14C-MBC was prepared using dry starch-g-polyacrylamide and starch-g-(acrylic acid-co-methyl methacrylate) N,N’-methyl bis-acrylamide, following the polymerization reaction at 85 °C for 30 min under N2 atmosphere to form the gelatinized starch. A predetermined quantity of carbendazim dissolved in acrylic acid was mixed with a small part of starch paste in a temperature-controlled water bath and stirred (300 rpm), and heated to 70 °C, was added by 0.05 g of AIBA(2’2-azobis[2-methylpropionamidine]dihydrochloride]). This reaction was proceeding for 5 h under a N2 atmosphere with reflux condensation to form the mixture A. Another part of starch was taken to synthesize the hydrogels (mixture B). The two mixtures were blended, and heated up to 70 °C until to obtain the rubbery product. Finally, the H-14C-MBC was dried, and sieved for incubation experiment25.
Incubation experiment and preparation of the bound residue
After 10 days pre-incubation, three 300 g aliquots of each soil was amended with SAP (0.5‰ (w/w)), and then were mixed with MBC at 4 mg kg−1. Subsequently, soil moisture content was regulated to 60% of water-holding capacity (WHC). Similarly, the SAP-free treatment with Carbendazim and SAP-encapsulated formulations underwent the same procedure. The incubation test was performed under aerobic conditions according to OECD (2002) guideline 30726. The fully mixed soils were transferred to 500-mL brown jars connected by a flow-through apparatus to the trapping solutions. All treatments were incubated at 25 ± 1 °C and ventilated periodically, and all absorption solutions were exchanged regularly with fresh solutions. At intervals of 0, 3, 6, 13, 20, 30, 45, 60, 80, and 100 d, three replicates of each treatment (10 g, dry weight equivalent) were collected.
Soil samples (10.0 g, dry weight) per treatment were extracted sequentially according to Helweg and Wang et al. with slight modification27,28. Briefly, soil samples were extracted three times with 30 mL of methanol/0.1 M hydrochloric acid solution (4:1, v/v), blended thoroughly, and shaken at 120 rpm for 2 h. After centrifugation at 6000 × g for 5 min, the deposits were similarly re-extracted by methanol, and ethyl acetate, consecutively, until no more 14C-radioactivity was detected in the extracts. The recovery extraction of 14C activity was approximately 95.52–101.65% when freshly spiked soils were analyzed. A 1-mL aliquot of every treatment supernatant was measured with addition of 10-mL cocktail A to measure the 14C-activity on LSC. The 14C-radioactivity of total extracted solvents was calculated as the extractable residue (ER). All remaining solutions were passed through a 0.22-μm filter and reduced in bulk to near dryness by a Vacuum Rotary Evaporator (Eyela SB-1000, Eyela, Tokyo, Japan) at 45 °C. The residue was re-dissolved in 10-mL methanol and condensed to 1-mL under a stream of nitrogen at ambient temperature for high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) analysis. All the post-extracted soils were air-dried. A homogenized soil sample of 1.0 g was combusted on the biological oxidizer and the released 14C-CO2 was trapped in 15 mL of cocktail B for analysis on LSC. The combustion recovery was 95.70 ± 1.42% (n = 3). The amount of 14C-radioactivity in the post-extracted soils was defined as the initial bound residue (BR).
Bioavailability experiment
Flowering Chinese cabbage was used for the bioavailability assay. The initial BR soil was mixed with fresh soils at the initial contents of 14C-BR of carbendazim (Table 3).
The uniformly mixed soil (50 g, dry weight equivalent) were placed in each 100-mL plastic pot for cultivation. The moisture of soil was adjusted to 60% of the soil WHC. Each germinated seeds were sown in each pot. Blank soils without 14C-BR soil were also planted with seeds as above. All treatments were incubated also under the same green conditions (25/20 °C, day/night; humidity, 80%; light, 16 h/8 h), with daily irrigation. The cabbage seedling for each treatment was harvested at 7, 21 and 35 days of exposure. The shoots and roots of seedling were separated. The roots were washed with tap water, and the height of plant was measured. All the plant parts were kept in paper envelope and dried at 60 °C to a constant weight. Aliquots of five dried plants were combusted on the biological oxidizer, and the released 14CO2 was absorbed in 15 mL liquid scintillation cocktail B. The radioactivity was measured by Quantulus 1220 ultra-low liquid scintillation spectrometer (ULLSS; Quantulus 1220, Perkinelmer, Turku, Finland) to estimate the amount of BR that was accumulated by the plant. The recovery efficiency of the above combustion procedure was 93.32 ± 1.41%.
Measurement of the released bound residue
After the cultivation of Cabbage, the soils were extracted by the same method. Aliquots of the final extract at each extraction step were transferred into 20-mL scintillation vials, and the 14C radioactivity was measured by LSC after addition of 10-mL scintillation cocktail A. Then all extracts were mixed together and condensed to near dryness on a vacuumed rotary evaporator (Eyela SB-1000, Eyela Co. Shanghai, China) at 40 °C. The residue was dissolved in 1.0 mL methanol, and the 14C-radioactivity of total extracted solvents was calculated as the extractable residue (ER). All the post-extracted soils were air-dried. 1.0 g homogenized soil sample was combusted on biological oxidizer and the released 14C-CO2 was trapped in 15 mL of cocktail B for analysis on LSC. The combustion recovery was 95.70 ± 1.42% (n = 3). The amount of 14C-radioactivity in the post-extracted soils was defined as bound residue (BR). The released rate of bound residue was calculated as initial BR minus the BR after the plant seeding, then divided by the initial BR.
Statistical analysis
All statistical analysis was performed using Origin 8.0 (Microcal Software, Northampton, MA) and SPSS 20.0 (IBM SPSS Statistics, Armonk, NY, U. S. A.). The significance was based on one-way ANOVA at α = 0.05. The data were presented as the mean ± standard derivation of three replicates.
References
Craven, A. Bound residues of organic compounds in the soil. Environmental Pollution 108, 15–18 (2000).
Zhu, X., Schroll, R., Dörfler, U. & Chen, B. Inoculation of soil with an Isoproturon degrading microbial community reduced the pool of “real non-extractable” Isoproturon residues. Ecotoxicology and Environmental Safety 149, 182–189 (2018).
Han, A. et al. Plant availability and phytotoxicity of soil bound residues of herbicide ZJ0273, a novel acetolactate synthase potential inhibitor. Chemosphere 77, 955–961 (2009).
Gao, Y., Wang, Y., Zeng, Y. & Zhu, X. Phytoavailability and Rhizospheric Gradient Distribution of Bound-Polycyclic Aromatic Hydrocarbon Residues in Soils. Soil Sci. Soc. Am. J. 77, 1572–1583 (2013).
Liu, X. et al. Bioavailability and release of nonextractable (bound) residues of chiral cycloxaprid using geophagous earthworm Metaphire guillelmi in rice paddy soil. Sci. Total Environ. 526, 243–250 (2015).
Guilherme, M. R. et al. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. European Polymer Journal 72, 365–385 (2015).
Alam, M. N. & Christopher, L. P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustainable Chemistry & Engineering 6, 8736–8742 (2018).
Ali, A. & Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 66, 6940–6967 (2018).
Sarkar, D. J. & Singh, A. Base triggered release of insecticide from bentonite reinforced citric acid crosslinked carboxymethyl cellulose hydrogel composites. Carbohydrate Polymers 156, 303–311 (2017).
Yi, Y. et al. Gelation of photocrosslinkable carboxymethyl chitosan and its application in controlled release of pesticide. Carbohydrate Polymers 86, 1007–1013 (2011).
Singh, B., Sharma, D. K. & Gupta, A. In vitro release dynamics of thiram fungicide from starch and poly(methacrylic acid)-based hydrogels. J. Hazard. Mater. 154, 278–286 (2008).
Yang, Y. et al. Effects of superabsorbent polymers on the fate of fungicidal carbendazim in soils. J. Hazard. Mater. 328, 70–79 (2017).
Yang, Y. et al. Superabsorbent hydrogels coating increased degradation and decreased bound residues formation of carbendazim in soil. Sci. Total Environ. 630, 1133–1142 (2018).
Ge, X., Huang, Z., Tian, S., Huang, Y. & Zeng, C. Complexation of carbendazim with hydroxypropyl-β-cyclodextrin to improve solubility and fungicidal activity. Carbohydrate Polymers 89, 208–212 (2012).
Delp, C. J. B and Related Fungicides. Modern Selective Fungicides: Properties, Applications, Mechanisms of Action, 233–244 (1987).
Lewandowska, A. & Walorczyk, S. Carbendazim Residues in the Soil and Their Bioavailability to Plants in Four Successive Harvests. Pol. J. Environ. Stud. 19, 757–761 (2010).
Zhang, R. B., Qiu, Z. X., Qiu, H. X. & Zhang, X. H. Frontal Polymerization of Superabsorbent Nanocomposites Based on Montmorillonite and Polyacrylic Acid with Enhanced Soil Properties. J. Appl. Polym. Sci. 131, 10 (2014).
Lerch, T. Z., Dignac, M. F., Nunan, N., Barriuso, E. & Mariotti, A. Ageing processes and soil microbial community effects on the biodegradation of soil C-13-2,4-D nonextractable residues. Environmental Pollution 157, 2985–2993 (2009).
Burauel, P. & Führ, F. Formation and long-term fate of non-extractable residues in outdoor lysimeter studies. Environmental Pollution 108, 45–52 (2000).
Li, X., He, J. Z., Liu, Y. R. & Zheng, Y. M. Effects of super absorbent polymers on soil microbial properties and Chinese cabbage (Brassica chinensis) growth. J. Soils Sediments 13, 711–719 (2013).
Li, X., He, J. Z., Hughes, J. M., Liu, Y. R. & Zheng, Y. M. Effects of super-absorbent polymers on a soil-wheat (Triticum aestivum L.) system in the field. Appl. Soil Ecol. 73, 58–63 (2014).
Gevao, B., Semple, K. T. & Jones, K. C. Bound pesticide residues in soils: a review. Environmental Pollution 108, 3–14 (2000).
Khodadadi Dehkordi, D. Evaluation of two types of superabsorbent polymer on soil water and some soil microbial properties. Paddy and Water. Environment 16, 143–152 (2018).
Zhang, S. F. et al. Synthesis and characterisation of starch grafted superabsorbent via 10 MeV electron-beam irradiation. Carbohydrate Polymers 101, 798–803 (2014).
Bai, C. et al. Starch-based hydrogel loading with carbendazim for controlled-release and water absorption. Carbohydrate Polymers 125, 376–383 (2015).
OECD Guidelines for the Testing of Chemicals: Aerobic and Anaerobic Transformation in Soil; No. 307; (Organization for Economic Co-Operation and Development (OECD), Paris; 2002).
Helweg, A. Degradation and adsorption of Carbendazim and 2-Aminobenzimidazole in soil. Pestic. Sci. 8, 71–78 (1977).
Wang, Z. C. et al. Biodegradation of carbendazim by a novel actinobacterium Rhodococcus jialingiae djl-6-2. Chemosphere 81, 639–644 (2010).
Acknowledgements
The financial funding for this research was provided by the National Key Research and Development Program of China (No.2016YFD0200201), and the National Natural Science Foundation of China (Nos. 21477105, 21507110 and 11275170), and China scholarship council, and the Fundamental Research Fund for the Central Universities.
Author information
Authors and Affiliations
Contributions
Y.Y. wrote the main manuscript text and conducted the experiments. W.L., Y.C., W.G. and X.G. helped process the samples. H.W. reviewed and modified the manuscript. Q.Y. reviewed this article and provided some suggestion for revision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Wang, H., Li, W. et al. Phytoavailability of bound residue of Carbendazim to Chinese cabbage (Brassica campestris ssp.chinensis) coexisted with Superabsorbent polymers. Sci Rep 10, 491 (2020). https://doi.org/10.1038/s41598-020-57488-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57488-8
This article is cited by
-
Mineralization and residue characteristics of chloramphenicol in aerobic soils: evidence from a carbon-14 study
Environmental Science and Pollution Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.